Abstract
Purpose
The objective of this study is to investigate the effect of 2, 5, and 20 % O2 on post-thaw day 3 human embryo culture until blastocyst stage.
Methods
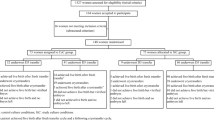
One hundred fifty-five day 3 human embryos were used. One hundred twenty out of 155 embryos were recovered after thawing. Surviving embryos were distributed into 2, 5, or 20 % O2 groups and cultured for 2.5 days. At the end of culture, blastocyst formation was assessed, and then, embryos were collected for RT-qPCR or immunofluorescence analysis.
Results
Using visible blastocoel to define blastocyst formation, 58.7 % (27/46) of surviving day 3 embryos formed blastocyst at 2 % O2, 63.6 % (28/44) at 5 % O2, and 66.7 % (20/30) at 20 % O2. The difference in blastocyst formation rates was not significant. Average blastocyst cell number was 119.44 ± 11.64 at 2 % O2, 142.55 ± 22.47 at 5 % O2, and 97.29 ± 14.87 at 20 % O2. Average apoptotic rate was 4.7 % ± 0.4 % for blastocyst formed at 2 % O2, 3.5 % ± 0.7 % at 5 % O2, and 5.8 % ± 1.1 % at 20 % O2. Apoptosis rate was significantly lower for blastocysts formed at 5 % O2 (p < 0.05). Compared with gene expression levels at 5 % O2, which were arbitrarily set as “1,” 20 % O2 is associated with significantly higher expression of BAX (2.14 ± 0.47), G6PD (2.92 ± 1.06), MnSOD (2.87 ± 0.88), and HSP70.1 (8.68 ± 4.19). For all genes tested, no significant differences were found between 2 and 5 % O2.
Conclusion
The result suggests that development of cryopreserved human embryos from day 3 to blastocyst stage benefits from culture at 5 % O2.



Similar content being viewed by others
References
Bavister B. Oxygen concentration and preimplantation development. Reprod Biomed Online. 2004;9(5):484–6.
Kirkegaard K, Hindkjaer JJ, Ingerslev HJ. Effect of oxygen concentration on human embryo development evaluated by time-lapse monitoring. Fertil Steril. 2013;99(3):738–44.e4.
Kasterstein E, Strassburger D, Komarovsky D, Bern O, Komsky A, Raziel A, et al. The effect of two distinct levels of oxygen concentration on embryo development in a sibling oocyte study. J Assist Reprod Genet. 2013;30(8):1073–9.
De Los Santos MJ, Gamiz P, Albert C, Galan A, Viloria T, Perez S, et al. Reduced oxygen tension improves embryo quality but not clinical pregnancy rates: a randomized clinical study into ovum donation cycles. Fertil Steril. 2013;100(2):402–7.
Bahceci M, Ciray HN, Karagenc L, Ulug U, Bener F. Effect of oxygen concentration during the incubation of embryos of women undergoing ICSI and embryo transfer: a prospective randomized study. Reprod Biomed Online. 2005;11(4):438–43.
Dumoulin JCM, Meijers CJJ, Bras M, Coonen E, Geraedts JPM, Evers JLH. Effect of oxygen concentration on human in-vitro fertilization and embryo culture. Hum Reprod. 1999;14(2):465–9.
Kovacic B, Vlaisavljevic V. Influence of atmospheric versus reduced oxygen concentration on development of human blastocysts in vitro: a prospective study on sibling oocytes. Reprod Biomed Online. 2008;17(2):229–36.
Meintjes M, Chantilis SJ, Douglas JD, Rodriguez AJ, Guerami AR, Bookout DM, et al. A controlled randomized trial evaluating the effect of lowered incubator oxygen tension on live births in a predominantly blastocyst transfer program dagger. Hum Reprod. 2009;24(2):300–7.
Bontekoe S, Mantikou E, van Wely M, Seshadri S, Repping S, Mastenbroek S. Low oxygen concentrations for embryo culture in assisted reproductive technologies. Cochrane Database Syst Rev. 2012 (7). doi:10.1002/14651858.Cd008950.Pub2.
Thompson JG, Simpson AC, Pugh PA, Donnelly PE, Tervit HR. Effect of oxygen concentration on in-vitro development of preimplantation sheep and cattle embryos. J Reprod Fertil. 1990;89(2):573–8.
Berthelot F, Terqui M. Effects of oxygen, CO2/pH and medium on the in vitro development of individually cultured porcine one- and two-cell embryos. Reprod Nutr Dev. 1996;36(3):241–51.
Quinn P, Harlow GM. The effect of oxygen on the development of preimplantation mouse embryos in vitro. J Exp Zool. 1978;206(1):73–80.
Feil D, Lane M, Roberts CT, Kelley RL, Edwards LJ, Thompson JG, et al. Effect of culturing mouse embryos under different oxygen concentrations on subsequent fetal and placental development. J Physiol. 2006;572(1):87–96.
Takahashi Y, Hishinuma M, Matsui M, Tanaka H, Kanagawa H. Development of in vitro matured/fertilized bovine embryos in a chemically defined medium: influence of oxygen concentration in the gas atmosphere. J Vet Med Sci. 1996;58(9):897–902.
Yuan YQ, Van Soom A, Coopman FO, Mintiens K, Boerjan ML, Van Zeveren A, et al. Influence of oxygen tension on apoptosis and hatching in bovine embryos cultured in vitro. Theriogenology. 2003;59(7):1585–96.
Harvey AJ, Kind KL, Pantaleon M, Armstrong DT, Thompson JG. Oxygen-regulated gene expression in bovine blastocysts. Biol Reprod. 2004;71(4):1108–19.
Thompson JG, McNaughton C, Gasparrini B, McGowan LT, Tervit HR. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J Reprod Fertil. 2000;118(1):47–55.
Li J, Foote RH. Culture of rabbit zygotes into blastocysts in protein-free medium with one to twenty per cent oxygen. J Reprod Fertil. 1993;98(1):163–7.
Leese HJ. Human embryo culture: back to nature. J Assist Reprod Genet. 1998;15(8):466–8.
Yedwab GA, Paz G, Homonnai TZ, David MP, Kraicer PF. The temperature, pH, and partial pressure of oxygen in the cervix and uterus of women and uterus of rats during the cycle. Fertil Steril. 1976;27(3):304–9.
Ottosen LD, Hindkaer J, Husth M, Petersen DE, Kirk J, Ingerslev HJ. Observations on intrauterine oxygen tension measured by fibre-optic microsensors. Reprod Biomed Online. 2006;13(3):380–5.
Diaz S, Ortiz ME, Croxatto HB. Studies on the duration of ovum transport by the human oviduct. III. Time interval between the luteinizing hormone peak and recovery of ova by transcervical flushing of the uterus in normal women. Am J Obstet Gynecol. 1980;137(1):116–21.
Liu HC, He ZY, Mele CA, Veeck LL, Davis O, Rosenwaks Z. Expression of apoptosis-related genes in human oocytes and embryos. J Assist Reprod Genet. 2000;17(9):521–33.
Wrenzycki C, Herrmann D, Carnwath JW, Niemann H. Alterations in the relative abundance of gene transcripts in preimplantation bovine embryos cultured in medium supplemented with either serum or PVA. Mol Reprod Dev. 1999;53(1):8–18.
Niemann H, Wrenzycki C. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: implications for subsequent development. Theriogenology. 2000;53(1):21–34.
Balasubramanian S, Son WJ, Kumar BM, Ock SA, Yoo JG, Im GS, et al. Expression pattern of oxygen and stress-responsive gene transcripts at various developmental stages of in vitro and in vivo preimplantation bovine embryos. Theriogenology. 2007;68(2):265–75.
Rizos D, Gutierrez-Adan A, Moreira P, O’Meara C, Fair T, Evans AC, et al. Species-related differences in blastocyst quality are associated with differences in relative mRNA transcription. Mol Reprod Dev. 2004;69(4):381–6.
Wrenzycki C, Herrmann D, Keskintepe L, Martins Jr A, Sirisathien S, Brackett B, et al. Effects of culture system and protein supplementation on mRNA expression in pre-implantation bovine embryos. Hum Reprod. 2001;16(5):893–901.
Rizos D, Lonergan P, Boland MP, Arroyo-Garcia R, Pintado B, de la Fuente J, et al. Analysis of differential messenger RNA expression between bovine blastocysts produced in different culture systems: implications for blastocyst quality. Biol Reprod. 2002;66(3):589–95.
Edgar DH, Bourne H, Speirs AL, McBain JC. A quantitative analysis of the impact of cryopreservation on the implantation potential of human early cleavage stage embryos. Hum Reprod. 2000;15(1):175–9.
Cutting R, Morroll D, Roberts SA, Pickering S, Rutherford A, Bfs, et al. Elective single embryo transfer: guidelines for practice British Fertility Society and Association of Clinical Embryologists. Hum Fertil (Camb). 2008;11(3):131–46.
Gardner DK, Schoolcraft WB. A randomized trial of blastocyst culture and transfer in in-vitro fertilization: reply. Hum Reprod. 1999;14(6):1663A.
Abruzzese RV, Fekete RA. Single cell gene expression analysis of pluripotent stem cells. Methods Mol Biol. 2013;997:217–24.
Li J, Smyth P, Cahill S, Denning K, Flavin R, Aherne S, et al. Improved RNA quality and TaqMan pre-amplification method (PreAmp) to enhance expression analysis from formalin fixed paraffin embedded (FFPE) materials. BMC Biotechnol. 2008;8:10.
Jedrusik A, Parfitt DE, Guo G, Skamagki M, Grabarek JB, Johnson MH, et al. Role of Cdx2 and cell polarity in cell allocation and specification of trophectoderm and inner cell mass in the mouse embryo. Genes Dev. 2008;22(19):2692–706.
Van Peer G, Mestdagh P, Vandesompele J. Accurate RT-qPCR gene expression analysis on cell culture lysates. Sci Rep. 2012;2:222.
Duran EM, Shapshak P, Worley J, Minagar A, Ziegler F, Haliko S, et al. Presenilin-1 detection in brain neurons and FOXp3 in peripheral blood mononuclear cells: normalizer gene selection for real time reverse transcriptase PCR using the deltadeltaCt method. Front Biosci. 2005;10:2955–65.
Pfister C, Pfrommer H, Tatagiba MS, Roser F. Detection and quantification of farnesol-induced apoptosis in difficult primary cell cultures by TaqMan protein assay. Apoptosis. 2013;18(4):452–66.
Byattsmith JG, Leese HJ, Gosden RG. An investigation by mathematical modeling of whether mouse and human preimplantation embryos in static culture can satisfy their demands for oxygen by diffusion. Hum Reprod. 1991;6(1):52–7.
Baltz JM, Biggers JD. Oxygen transport to embryos in microdrop cultures. Mol Reprod Dev. 1991;28(4):351–5.
Clark AR, Stokes YM, Lane M, Thompson JG. Mathematical modelling of oxygen concentration in bovine and murine cumulus-oocyte complexes. Reproduction. 2006;131(6):999–1006.
Saibil HR. Biochemistry. Machinery to reverse irreversible aggregates. Science. 2013;339(6123):1040–1.
Riley JK, Moley KH. Glucose utilization and the PI3-K pathway: mechanisms for cell survival in preimplantation embryos. Reproduction. 2006;131(5):823–35.
Heilig C, Brosius F, Siu B, Concepcion L, Mortensen R, Heilig K, et al. Implications of glucose transporter protein type 1 (GLUT1)-haplodeficiency in embryonic stem cells for their survival in response to hypoxic stress. Am J Pathol. 2003;163(5):1873–85.
Dan-Goor M, Sasson S, Davarashvili A, Almagor M. Expression of glucose transporter and glucose uptake in human oocytes and preimplantation embryos. Hum Reprod. 1997;12(11):2508–10.
Kind KL, Collett RA, Harvey AJ, Thompson JG. Oxygen-regulated expression of GLUT-1, GLUT-3, and VEGF in the mouse blastocyst. Mol Reprod Dev. 2005;70(1):37–44.
Wrenzycki C, Herrmann D, Niemann H. Timing of blastocyst expansion affects spatial messenger RNA expression patterns of genes in bovine blastocysts produced in vitro. Biol Reprod. 2003;68(6):2073–80.
Bloor DJ, Wilson Y, Kibschull M, Traub O, Leese HJ, Winterhager E, et al. Expression of connexins in human preimplantation embryos in vitro. Reprod Biol Endocrinol. 2004;2:25.
Houghton FD. Role of gap junctions during early embryo development. Reproduction. 2005;129(2):129–35.
Brison DR, Leese HJ. Blastocoel cavity formation by preimplantation rat embryos in the presence of cyanide and other inhibitors of oxidative phosphorylation. J Reprod Fertil. 1994;101(2):305–9.
Zhou S, Xie Y, Puscheck EE, Rappolee DA. Oxygen levels that optimize TSC culture are identified by maximizing growth rates and minimizing stress. Placenta. 2011;32(6):475–81.
Xie Y, Zhou S, Jiang Z, Dai J, Puscheck EE, Lee I, et al. Hypoxic stress induces, but cannot sustain trophoblast stem cell differentiation to labyrinthine placenta due to mitochondrial insufficiency. Stem Cell Res. 2014;13(3 Pt A):478–91.
Houghton FD. Energy metabolism of the inner cell mass and trophectoderm of the mouse blastocyst. Differentiation. 2006;74(1):11–8.
Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102(13):4783–8.
Wale PL, Gardner DK. Time-lapse analysis of mouse embryo development in oxygen gradients. Reprod Biomed Online. 2010;21(3):402–10.
Heo YS, Cabrera LM, Bormann CL, Shah CT, Takayama S, Smith GD. Dynamic microfunnel culture enhances mouse embryo development and pregnancy rates. Hum Reprod. 2010;25(3):613–22.
Acknowledgments
Thanks to Yuliang Liu (BS) and Yanhong Zeng (BS) for the technical support, Baomin Lu (BS) for the preparation of culture medium, Dr Tao Li for the advice on experimental design, and performance, and to Drs Husam Abu-Soud, Maik Huttemann, Mark Kibschull, and Michael Diamond for the analysis and comments on the manuscript as well as Rappolee lab members Drs Quanwen Li and Mohammed Abdulhasan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Written informed consent was obtained from all participants before the usage of their embryos. The institutional review board (IRB) of the First Affiliated Hospital of Sun Yat-Sen University approved the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This research was supported by grants from National Basic Research Program of China (No. 2012CB947604), Science and Information Technology of Guangzhou (No. 201300000097), DAR and EEP from the Office of the Vice President for Research at Wayne State University, and from the funding of the Mary Iacobelli Endowed Chair.
Additional information
Capsule The result suggests that development of cryopreserved human embryos from day 3 to blastocyst stage benefits from culture at 5 % O2.
Yu Yang and Yanwen Xu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, Y., Xu, Y., Ding, C. et al. Comparison of 2, 5, and 20 % O2 on the development of post-thaw human embryos. J Assist Reprod Genet 33, 919–927 (2016). https://doi.org/10.1007/s10815-016-0693-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0693-5