Abstract
Purpose
To study the effect of α-linolenic acid (ALA) on meiotic maturation, mRNA abundance of apoptosis-related (Bax and Bcl-2) molecules, and blastocyst formation in ovine oocytes.
Methods
A preliminary experiment was conducted to analyze the concentration of ALA in “small” (≤2 mm) and “large” (≥6 mm) follicles using gas chromatography/mass spectrometry analysis. The concentration of ALA in small and large follicles was determined to be in a range of 75.4 to 125.7 μM, respectively. In vitro maturation (IVM) of oocyte was then performed in presence of 0 (control), 10 (ALA-10), 50 (ALA-50), 100 (ALA-100), and 200 (ALA-200) μM of ALA. Meiotic maturation and mRNA abundance of Bax, and Bcl-2 genes was evaluated after 24 h of IVM. The embryonic cleavage and blastocyst formation following parthenogenetic activation were also determined for each group.
Results
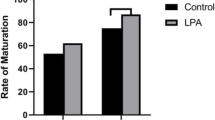
The highest concentration of ALA (ALA-200) decreased the oocyte maturation rate compared with the control group. Analysis of apoptosis-related genes in oocytes after IVM revealed lesser transcript abundances for Bax gene, and higher transcript abundances for Bcl-2 gene in ALA-treated oocytes as compared with the control oocytes. In term of cleavage rate (considered as 2-cell progression), we did not observe any differences among the groups. However, ALA-100 group promoted more blastocyst formation as compared with the control group.
Conclusion
Our results suggested that ALA treatment during IVM had a beneficial effect on developmental competence of ovine oocytes by increasing the blastocyst formation and this might be due to the altered abundance of apoptosis-regulatory genes.


Similar content being viewed by others
References
McKeegan PJ, Sturmey RG. The role of fatty acids in oocyte and early embryo development. Reprod Fertil Dev. 2012;24(1):59–67. doi:10.1071/RD11907.
Leroy JL, Vanholder T, Mateusen B, Christophe A, Opsomer G, de Kruif A, et al. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction. 2005;130(4):485–95. doi:10.1530/rep.1.00735.
Marei WF, Wathes DC, Fouladi-Nashta AA. The effect of linolenic Acid on bovine oocyte maturation and development. Biol Reprod. 2009;81(6):1064–72. doi:10.1095/biolreprod.109.076851.
Marei WF, Wathes DC, Fouladi-Nashta AA. Impact of linoleic acid on bovine oocyte maturation and embryo development. Reproduction. 2010;139(6):979–88. doi:10.1530/REP-09-0503.
Wonnacott KE, Kwong WY, Hughes J, Salter AM, Lea RG, Garnsworthy PC, et al. Dietary omega-3 and −6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryos. Reproduction. 2009;139(1):57–69. doi:10.1530/REP-09-0219.
Bender K, Walsh S, Evans AC, Fair T, Brennan L. Metabolite concentrations in follicular fluid may explain differences in fertility between heifers and lactating cows. Reproduction. 2010;139(6):1047–55. doi:10.1530/REP-10-0068.
Fouladi-Nashta AA, Wonnacott KE, Gutierrez CG, Gong JG, Sinclair KD, Garnsworthy PC, et al. Oocyte quality in lactating dairy cows fed on high levels of n-3 and n-6 fatty acids. Reproduction. 2009;138(5):771–81. doi:10.1530/REP-08-0391.
Childs S, Hennessy AA, Sreenan JM, Wathes DC, Cheng Z, Stanton C, et al. Effect of level of dietary n-3 polyunsaturated fatty acid supplementation on systemic and tissue fatty acid concentrations and on selected reproductive variables in cattle. Theriogenology. 2008;70(4):595–611. doi:10.1016/j.theriogenology.2008.04.002.
Funston RN. Fat supplementation and reproduction in beef females. J Anim Sci. 2004;82(E-Suppl):E154–61.
Staples CR, Burke JM, Thatcher WW. Influence of supplemental fats on reproductive tissues and performance of lactating cows. J Dairy Sci. 1998;81(3):856–71. doi:10.3168/jds.S0022-0302(98)75644-9.
Sturmey RG, Reis A, Leese HJ, McEvoy TG. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod Domest Anim. 2009;44 Suppl 3:50–8. doi:10.1111/j.1439-0531.2009.01402.x.
Santos JE, Bilby TR, Thatcher WW, Staples CR, Silvestre FT. Long chain fatty acids of diet as factors influencing reproduction in cattle. Reprod Domest Anim. 2008;43 Suppl 2:23–30. doi:10.1111/j.1439-0531.2008.01139.x.
Van Hoeck V, Leroy JL, Arias Alvarez M, Rizos D, Gutierrez-Adan A, Schnorbusch K, et al. Oocyte developmental failure in response to elevated nonesterified fatty acid concentrations: mechanistic insights. Reproduction. 2013;145(1):33–44. doi:10.1530/REP-12-0174.
Barcelo-Coblijn G, Murphy EJ. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog Lipid Res. 2009;48(6):355–74. doi:10.1016/j.plipres.2009.07.002.
Ghaffarilaleh V, Fouladi-Nashta A, Paramio MT. Effect of alpha-linolenic acid on oocyte maturation and embryo development of prepubertal sheep oocytes. Theriogenology. 2014;82(5):686–96. doi:10.1016/j.theriogenology.2014.05.027.
O’Fallon JV, Busboom JR, Nelson ML, Gaskins CT. A direct method for fatty acid methyl ester synthesis: application to wet meat tissues, oils, and feedstuffs. J Anim Sci. 2007;85(6):1511–21. doi:10.2527/jas.2006-491.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi:10.1006/meth.2001.1262.
Lan GC, Han D, Wu YG, Han ZB, Ma SF, Liu XY, et al. Effects of duration, concentration, and timing of ionomycin and 6-dimethylaminopurine (6-DMAP) treatment on activation of goat oocytes. Mol Reprod Dev. 2005;71(3):380–8. doi:10.1002/mrd.20267.
Andrade LN, de Lima TM, Curi R, Castrucci AM. Toxicity of fatty acids on murine and human melanoma cell lines. Toxicol In Vitro. 2005;19(4):553–60. doi:10.1016/j.tiv.2005.02.002.
Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2003;7(3):249–57.
Ruvolo PP, Deng X, May WS. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001;15(4):515–22.
Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13(15):1899–911.
Marei WF, Wathes DC, Fouladi-Nashta AA. Differential effects of linoleic and alpha-linolenic fatty acids on spatial and temporal mitochondrial distribution and activity in bovine oocytes. Reprod Fertil Dev. 2012;24(5):679–90.
Haberman Y, Alon LT, Eliyahu E, Shalgi R. Receptor for activated C kinase (RACK) and protein kinase C (PKC) in egg activation. Theriogenology. 2011;75(1):80–9.
Al Darwich A, Perreau C, Petit MH, Papillier P, Dupont J, Guillaume D, et al. Effect of PUFA on embryo cryoresistance, gene expression and AMPKalpha phosphorylation in IVF-derived bovine embryos. Prostaglandins Other Lipid Mediat. 2010;93(1–2):30–6. doi:10.1016/j.prostaglandins.2010.06.002.
Cha KY, Chian RC. Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update. 1998;4(2):103–20.
Elvin JA, Yan C, Matzuk MM. Growth differentiation factor-9 stimulates progesterone synthesis in granulosa cells via a prostaglandin E2/EP2 receptor pathway. Proc Natl Acad Sci U S A. 2000;97(18):10288–93. doi:10.1073/pnas.180295197 180295197.
Acknowledgments
This work was supported by a grant from the Stem Cell Technology Research Centre, Tehran, Iran. The authors thank the members of their own laboratories for their helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule α-Linolenic acid in ovine oocyte maturation medium has been found to affect developmental competence, and expression of the Bax, and Bcl-2 genes
Rights and permissions
About this article
Cite this article
Veshkini, A., Asadi, H., Khadem, A.A. et al. Effect of Linolenic acid during in vitro maturation of ovine oocytes: embryonic developmental potential and mRNA abundances of genes involved in apoptosis. J Assist Reprod Genet 32, 653–659 (2015). https://doi.org/10.1007/s10815-015-0439-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0439-9