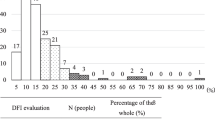
Abstract
Objective
To evaluate the relationship between sperm nuclear vacuoles and sperm morphology and to investigate the influence of the rate of spermatozoa with head vacuolization (SVR) in a seminal sample on the clinical outcomes in couples undergoing intracytoplasmic sperm injection.
Materials
26 patients undergoing infertility investigations were included and were divided in two groups according to an SVR ≤ 20,28 % (Group A) or > 20,28 % (Group B), and were investigated to verify the influence of SVR on the fertilization rate, embryo quality, pregnancy and implantation rates.
Results
Abnormal spermatozoa with nuclear vacuoles were significantly higher (p < 0.001) than the percentage of normal spermatozoa with nuclear vacuoles. Patients in group A had a percentage of abnormal sperm with nuclear vacuole significantly lower compared to group B (p < 0,001), but there was no difference in the percentage of normal sperm with nuclear vacuoles. Fertilization rates and the number of top quality embryos did not differ between the two groups. The pregnancy and implantation rates were significantly higher in Group A compared to Group B (respectively p < 0,05 and p < 0.001).
Conclusions
For the first time, we propose a cut off value in the proportion of sperms with nuclear vacuolization on the total of sperm in seminal samples, and demonstrate a relationship between SNV and clinical outcomes after ICSI. The SNV rate could be introduced as an easy diagnostic evaluation prior to perform an ICSI cycle.


Similar content being viewed by others
References
Akl LD, Oliveira JB, Petersen CG, Mauri AL, Silva LF, Massaro FC, Baruffi RL, Cavagna M, Franco Jr JG. Efficacy of the motile sperm organelle morphology examination (MSOME) in predicting pregnancy after intrauterine insemination. Reprod Biol Endocrinol. 2011;9:120.
Bartoov B, Eltes F, Pansky M, Lederman H, Caspi E, Soffer Y. Estimating fertility potential via semen analysis data. Hum Reprod. 1993;8(1):65–70.
Bartoov B, Eltes F, Pansky M, Langzam J, Reichart M, Soffer Y. Improved diagnosis of male fertility potential via a combination of quantitative ultramorphology and routine semen analyses. Hum Reprod. 1994;9(11):2069–75.
Bartoov B, Berkovitz A, Eltes F. Selection of spermatozoa with normal nuclei to improve pregnancy rate with intracitoplasmatic sperm injection. N Engl J Med. 2001;345:1067–8.
Bartoov B, Berkovitz A, Eltes F, Kogosowski A, Menezo Y, Barak Y. Real-time fine morphology of motile sperm cells is asociated with IVF-ICSI outcome. J Androl. 2002;23:1–8.
Bartoov B, Berkoviz A, Eltes F, Kogosowski A, Yogoda A, Lederman H, Artzi S, Gross M, Barak Y. Pregnancy rate are higher with intracitoplsmatic morphologically selected sperm injection than with conventional intracytoplasmatic injection. Fertil Steril. 2003;80:1413–9.
Berkovitz A, Eltes F, Soffer Y, Zabludovsky N, Beyth Y, Farhi J, Levran D, Bartoov B. ART success and in vivo sperm cell selection depend on the ultramorphological status of spermatozoa. Andrologia. 1999;31:1–8.
Berkovitz A, Eltes F, Yaari S, Katz N, Barr I, Fishman A, Bartoov B. The morphological normalcy of the sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Hum Reprod. 2005;20:185–90.
Berkoviz A, Eltes F, Ellenbogen A, Peer S, Feldberg D, Bartoov B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum Reprod. 2006;21:1787–90.
Boitrelle F, Ferfouri F, Petit JM, Segretain D, Tourain C, Bergere M, Bailly M, Vialard F, Albert M, Selva J. Large human sperm vacuoles observed in motile spermatozoa under high magnification: nuclear thumbprint linked to failure of chromatin condensation. Hum Reprod. 2011;26:1650–8.
Cassuto NG, Bouret D, Plouchart JM, Jellard S, Vanderzwalmen P, Balet R, Larue L, Barak Y. A new real-time morphology classification for human spermtozoa: a link for fertilization and improved embryo quality. Fertil Steril. 2009;92:1616–25.
De Vos A, Van De Velde H, Joris H, Verheyen G, Devroey P, Van Steirteghem A. Influence of individual sperm morphology on fertilization, embryo morphology, and pregnancy outcome of intracytoplasmic sperm injection. Fertil Steril. 2003;79(1):42–8.
Franco Jr JG, Baruffi RLB, Mauri AL, Petersen CG, Oliveira JBA, Vagnini L. Significance of large nuclear vacuoles in human spermatozoa: implications for ICSI. Reprod Biomed Online. 2008;17:42–5.
Franco Jr JG, Mauri AL, Petersen CG, Massaro FC, Silva LF, Felipe V, Cavagna M, Pontes A, Baruffi LR, Oliveira JBA, Vagnini LD. Large nuclear vacuoles are indicative of abnormal chromatine packaging in human spermatozoa. Int J Androl. 2012;35(1):46–51.
Garolla A, Fortini D, Menegazzo M, De Toni L, Nicoletti V, Moretti A, Selice R, Engl B, Foresta C. High-power microscopy for selecting spermatozoa for ICSI by physiological status. Reprod Biomed Online. 2008;17:610–6.
Gianaroli L, Magli MC, Ferraretti AP, Crippa A, Lappi M, Capitani S, Baccetti B. Birefringence characteristics in sperm heads allow for the selection of reacted spermatozoa for intracytoplasmic sperm injection. Fertil Steril. 2010;93(3):807–13.
Junca A, Cohen-Bacrie M, Hazout PA. Improvement of fertilization and pregnancy rate after intracytoplasmic fine morphology selected sperm injection. Fertil Steril. 2004;82:S173.
Kacem O, Sifer C, Barraud-Lange V, Ducot B, De Ziegler D, Poirot C, Wolf J. Sperm nuclear vacuoles, as assessed by motile sperm organellar morphological examination, are mostly of acrosomal origin. Reprod Biomed Online. 2010;20:132–7.
Kruger TF, Swanson RJ, Hamilton M, Simmons KF, Acosta AA, Matta JF, Oehinger S, Morshedi M. Abnormal sperm morphology and other semen parameters related to the outcome of the hamster oocyte human sperm penetration assay. Int J Androl. 1988;11(2):107–13.
Oliveira JB, Massaro FC, Mauri AL, Petersen CG, Nicoletti AP, Baruffi RL, Franco Jr JG. Motile sperm organelle morphology examination is stricter than Tygerberg criteria. Reprod Biomed Online. 2009;18(3):320–6.
Ombelet W, Bosmans E, Janssen M, Cox A, Vlasselaer J, Gyselaere W, Vandeput H, Gielen J, Pollet H, Maes M, Steeno O, Kruger T. Semen parameters in a fertile versus subfertile population: a need for change in the interpretation of semen testing. Hum Reprod. 1997;12(5):987–93.
Palermo F, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–8.
Perdrix A, Travers A, Chelli MH, Escalier D, Do Rego JL, Milazzo JP, Mousset-Siméon N, Macé B, Rives N. Assessment of acrosome and nuclear abnormalities in human spermatozoa with large vacuoles. Hum Reprod. 2011;26:47–58.
Petersen CG, Massaro FC, Mauri AL, Oliveira JB, Baruffi RL, Franco Jr JG. Efficacy of hyaluronic acid binding assay in selecting motile spermatozoa with normal morphology at high magnification. Reprod Biol Endocrinol. 2010;3(8):149.
Razavi SH, Nasr-Esfahani MH, Deemeh MR, Shayesteh M, Tavalee M. Evaluation of zeta and HA-binding methods for selection of spermatozoa with normal morphology, protamine content and DNA integrity. Andrologia. 2010;42(1):13–9.
Saïdi R, Rives N, Gruel E, Mazurier S, Mousset-Siméon N, Macé B. Nouvelle classification du spermocytogramme à fort grossissement. Méd Reprod Gyn Endo. 2008;10:315–24.
Sermondade N, Vialard F, Bergère M, Hammound I, Cavelot P, Selva J, Albert M. Evaluation de l’apport de la méthode d’observation des spermatoides à fort groissessement eh ICSI. Andrologie. 2007;17:212–21.
Taşdemir I, Taşdemir M, Tavukçuoglu S, Kahraman S, Biberoģlu K. Effect of abnormal sperm head morphology on the outcome of intracytoplasmic sperm injection in humans. Hum Reprod. 1997;12(6):1214–7.
van der Merwe FH, Kruger TF, Oehninger SC, Lombard CJ. The use of semen parameters to identify the subfertile male in the general population. Gynecol Obstet Invest. 2005;59(2):86–91.
Vanderzwalmet P, Hiemer A, Rubner P, Bach M, Neyer A, Stecher A, Uher P, Zint M, Lejeune B, Vanderzwalmet S, et al. Blastocyst development after sperm selection at high magnification is associated with size and number of nuclear vacuoles. Reprod Biomed Online. 2008;17:617–27.
Veeck LL. Atlas of the human oocyte and early conceptus. Baltimore: Williams & Wilkins; 1986.
Watanabe S, Tanaka A, Fujii S, Mizunuma H, Fukui A, Fukuhara R, Nakamura R, Yamada K, Tanaka I, Awata S, Nagayoshi M. An investigation of the potential effect of vacuoles in human sperm on DNA damage using a chromosome assay and the TUNEL assay. Hum Reprod. 2011;26(5):978–86.
World Health Organization. WHO laboratory manual for examination and processing of human semen 5th ed Cambridge. UK: Cambridge University Press; 2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule We propose a cut off value in the proportion of sperm with nuclear vacuolization (SNV) on the total of sperm in seminal samples, and demonstrate a relationship between SNV and clinical outcomes after ICSI. The SNV rate could be introduced as an easy diagnostic evaluation prior to perform an ICSI cycle.
Rights and permissions
About this article
Cite this article
Falagario, D., Brucculeri, A.M., Depalo, R. et al. Sperm head vacuolization affects clinical outcome in ICSI cycle. A proposal of a cut-off value. J Assist Reprod Genet 29, 1281–1287 (2012). https://doi.org/10.1007/s10815-012-9858-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9858-z