Abstract
Purpose
Cryopreservation of a complete ovary may be a future method for fertility preservation in cancer patients. Difficulties exist in cryopreservation of the relatively large ovarian tissue mass. This study evaluates whether a human postmenopausal ovary can be used, as a complement to animal models, in studies of this research field.
Methods
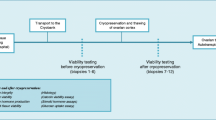
Postmenopausal human ovaries (n = 10) were isolated and flushed through ovarian arteries with either the cryoprotectant dimethylsulphoxide or Ringer-Acetate, followed by slow freezing. After thawing, production of androgens during in vitro perfusion and morphology (light/electron microscopy) were assessed.
Results
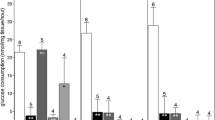
The dimethylsulphoxide-cryopreserved ovaries showed larger secretion of androgens during perfusion than Ringer Acetate-cryopreserved ovaries. Light microscopy showed well preserved morphology in both groups. Electron microscopy revealed normal appearance of stroma and vessels in the dimethylsulphoxide group.
Conclusions
The study demonstrates the potential to use the postmenopausal human ovary for further studies aiming at optimizing cryopreservation protocols, with special reference to ovarian vascularity and stroma.





Similar content being viewed by others
References
Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31.
Cobo A, Bellver J, Domingo J, Perez S, Crespo J, Pellicer A, et al. New options in assisted reproduction technology: the Cryotop method of oocyte vitrification. Reprod Biomed Online. 2008;17:68–72.
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10.
Oktay K, Oktem O. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience. Fertil Steril. 2010;93:762–8.
Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 C. Endocrinology. 1999;140:462–71.
Bedaiwy MA, Hussein MR, Biscotti C, Falcone T. Cryopreservation of intact human ovary with its vascular pedicle. Hum Reprod. 2006;21:3258–69.
Hilders CG, Baranski AG, Peters L, Ramkhelawan A, Trimbos JB. Successful human ovarian autotransplantation to the upper arm. Cancer. 2004;101:2771–8.
Leporrier M, von Theobald P, Roffe JL, Muller G. A new technique to protect ovarian function before pelvic irradiation. Heterotopic ovarian autotransplantation. Cancer. 1987;60:2201–4.
Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med. 2008;359:2617–8.
Donnez J, Jadoul P, Squifflet J, Van Langendonckt A, Donnez O, Van Eyck AS, et al. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24:87–100.
Hickey M, Ambekar M, Hammond I. Should the ovaries be removed or retained at the time of hysterectomy for benign disease? Hum Reprod Update. 2010;16:131–41.
Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrere S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril. 2004;82:1390–4.
Martinez-Madrid B, Camboni A, Dolmans MM, Nottola S, Van Langendonckt A, Donnez J. Apoptosis and ultrastructural assessment after cryopreservation of whole human ovaries with their vascular pedicle. Fertil Steril. 2007;87:1153–65.
Jadoul P, Donnez J, Dolmans MM, Squifflet J, Lengele B, Martinez-Madrid B. Laparoscopic ovariectomy for whole human ovary cryopreservation: technical aspects. Fertil Steril. 2007;87:971–5.
Brannstrom M, Johansson BM, Sogn J, Janson PO. Characterization of an in vitro perfused rat ovary model: ovulation rate, oocyte maturation, steroidogenesis and influence of PMSG priming. Acta Physiol Scand. 1987;130:107–14.
Gerritse R, Beerendonk CC, Tijink MS, Heetkamp A, Kremer JA, Braat DD, et al. Optimal perfusion of an intact ovary as a prerequisite for successful ovarian cryopreservation. Hum Reprod. 2008;23:329–35.
Bromer JG, Patrizio P. Fertility preservation: the rationale for cryopreservation of the whole ovary. Semin Reprod Med. 2009;27:465–71.
Wranning CA, Akhi SN, Kurlberg G, Brannstrom M. Uterus transplantation in the rat: model development, surgical learning and morphological evaluation of healing. Acta Obstet Gynecol Scand. 2008;87:1239–47.
Qi S, Ma A, Xu D, Daloze P, Chen H. Cryopreservation of vascularized ovary: an evaluation of histology and function in rats. Microsurgery. 2008;28:380–6.
Onions VJ, Webb R, McNeilly AS, Campbell BK. Ovarian endocrine profile and long-term vascular patency following heterotopic autotransplantation of cryopreserved whole ovine ovaries. Hum Reprod. 2009;24:2845–55.
Yin H, Wang X, Kim SS, Chen H, Tan SL, Gosden RG. Transplantation of intact rat gonads using vascular anastomosis: effects of cryopreservation, ischaemia and genotype. Hum Reprod. 2003;18:1165–72.
Imhof M, Bergmeister H, Lipovac M, Rudas M, Hofstetter G, Huber J. Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. Fertil Steril. 2006;85 Suppl 1:1208–15.
Janson PO, LeMaire WJ, Kallfelt B, Holmes PV, Cajander S, Bjersing L, et al. The study of ovulation in the isolated perfused rabbit ovary. 1. Methodology and pattern of steroidogenesis. Biol Reprod. 1982;26:456–65.
Abrahamsson G, Janson PO, Kullander S. An in vitro perfusion method for metabolic studies on human postmenopausal ovaries. Acta Obstet Gynecol Scand. 1990;69:527–32.
Adashi EY. The climacteric ovary as a functional gonadotropin-driven androgen-producing gland. Fertil Steril. 1994;62:20–7.
Couzinet B, Meduri G, Lecce MG, Young J, Brailly S, Loosfelt H, et al. The postmenopausal ovary is not a major androgen-producing gland. J Clin Endocrinol Metab. 2001;86:5060–6.
Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92:3040–3.
Milenkovic M, Wallin A, Ghahremani M, Brannstrom M: Whole sheep ovary cryopreservation: evaluation of a slow freezing protocol with dimethylsulphoxide. J Assist Reprod Genet 2010;
Kim SS, Yang HW, Kang HG, Lee HH, Lee HC, Ko DS, et al. Quantitative assessment of ischemic tissue damage in ovarian cortical tissue with or without antioxidant (ascorbic acid) treatment. Fertil Steril. 2004;82:679–85.
Hreinsson J, Zhang P, Swahn ML, Hultenby K, Hovatta O. Cryopreservation of follicles in human ovarian cortical tissue. Comparison of serum and human serum albumin in the cryoprotectant solutions. Hum Reprod. 2003;18:2420–8.
Acknowledgments
Supported by Division of Obstetrics and Gynecology, Telemark Hospital, Norway and by grants from: Swedish Research Council, Stockholm, Sweden; Sahlgrenska Academy ALF, Göteborg, Sweden; Hjalmar Svensson’s Research Foundation, Göteborg, Sweden; and Assar Gabrielsson’s Research Foundation, Göteborg, Sweden
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
The study evaluates the use of human postmenopausal ovaries for ovarian cryopreservation research.
Rights and permissions
About this article
Cite this article
Milenkovic, M., Gharemani, M., Bergh, A. et al. The human postmenopausal ovary as a tool for evaluation of cryopreservation protocols towards whole ovary cryopreservation. J Assist Reprod Genet 28, 453–460 (2011). https://doi.org/10.1007/s10815-011-9547-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-011-9547-3