Abstract
Purpose
The aim of this study is to determine whether inclusion of caspase inhibitor can improve the efficacy of cryopreservation of ovarian tissue.
Methods
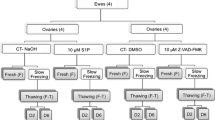
Mice were randomly assigned to the Group A (fresh control group) Group B (inclusion of caspase inhibitor) and Group C (non-inclusion of caspase inhibitor). Ovarian tissue in Group B and Group C was vitrified-thawed. TUNEL assay and Bax protein detection were measured after cryopreservation. The mice in all groups received autotransplantation. The number of days before the resumption of estrous cycles was measured daily from the 5th day after surgery, and the percentage of cells expressing PCNA in grafts was measured one month following transplantation.
Results
The incidence of TUNEL positive follicles in Group B was significantly higher than that in Group C. Similarly, the percentage of follicles expressing Bax protein in Group B was significantly higher than that in Group C. The number of days before the resumption of estrous cycles in Group B was significantly less than that in Group C. In addition, the percentage of follicular and stromal cells expressing PCNA of grafts in Group B was significantly higher than that in Group C.
Conclusions
The global caspase inhibitor Z-VAD-FMK decreases the incidence of apoptosis of ovarian tissue induced by cryopreservation, and inclusion of caspase inhibitor improves the efficacy of cryopreservation of ovarian tissue.



Similar content being viewed by others
References
Baker TG. Radiosensitivity of mammalian oocytes with particular reference to the human female. Am J Obstet Gynecol. 1971;110:746–61.
Apperley JF, Reddy N. Mechanism and management of treatment-related gonadal failure in recipients of high dose chemoradiotherapy. Blood Rev. 1995;9:93–116.
Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001;286:1490–3.
Baird DT, Webb R, Campbell BK, Harkness LM, Gsden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196°C. Endocrinology. 1999;140:462–71.
Rimon E, Cohen T, Dantes A. Apoptosis in cryopreserved human ovarian tissue obtained from cancer patients: a tool for evaluating cryopreservation utility. Int J Oncol. 2005;27:345–53.
Tirelli M, Basini G, Grasselli F, Bianco F, Tamanini C. Cryopreservation of pig granulosa cells: effect of FSH addition to freezing medium. Domest Anim Endocrinol. 2005;28:17–33.
Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–70.
Rugh R. The mouse: its reproduction and development. New York: Oxford University Press; 1990. p. 38–9.
Torrents E, Boiso I, Barri PN. Applications of ovarian tissue transplantation in experimental biology and medicine. Hum Reprod Update. 2003;9:471–81.
Gosden RG. Low temperature storage and grafting of human ovarian tissue. Mol Cell Endocrinol. 2000;163:125–9.
Nugent D, Neirow D, Brook PE, Aubard Y, Gosden RG. Transplantation in reproductive medicine: previous experience, present knowledge and future prospects. Hum Reprod Update. 1997;3:267–80.
Gao D, Critser JK. Mechanisms of cryoinjury in living cells. ILAR. 2000;41:181–96.
Mazur P. Freezing of living cells: mechanism and implications. Am J Physiol. 1984;247:C125–42.
Fauqe P, Amor AB, Joanne CJ, Agnani G. Use of trypan blue staining to assess the quality of ovarian cryopreservation. Fertil Steril. 2007;87:1200–7.
Onions VJ, Mitchell MRP, Campbell BK, Webb R. Ovarian tissue viability following whole ovine ovary cryopreservation: assessing the effects of sphingosine-1-phosphate inclusion. Hum Reprod. 2008;23:606–18.
Shin SY, Lee JY, Lee EY. Protective effect of vascular endothelial growth factor (VEGF) in frozen-thawed granulosa cells is mediated by inhibition of apoptosis. Eur J Obstet Gynecol Reprod Biol. 2006;125:233–8.
Morita Y, Perez GI, Paris F. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109–14.
Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly TL. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med. 1997;3:1228–32.
Haimovitz-Friedman A, Kan CC, Ehleiter D, Persud RS, McLoughlin M, Fuks Z, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525–35.
Alnemri ES. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J Cell Biochem. 1997;64:33–42.
Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12.
Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–42.
Thornberry N, Lazebnik Y. Caspase: enimies within. Science. 1998;281:1312–6.
Earnshaw WC. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Bio Chem. 1999;68:383–424.
Slee EA. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 1999;6:1074–87.
Budihardjo I. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–90.
Liu X. DEF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–84.
Simizu S. Requirement of caspase-3(-like)protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J Biol Chem. 1998;273:26900–7.
Zheng T. Caspase-3 controls both cytoplasmic and nuclear events associated with Fas-mediated apoptosis in vivo. Proc Natl Acad Sci USA. 1998;95:13618–23.
Gottlieb RA, Gruol DL, Zhu JY. Preconditioning rabbit cardiomyocytes: role of pH, vacuolar proton ATPase, and apoptosis. J Clin Invest. 1996;97:2391–8.
Himi T, Ishizaki Y, Murota SI. A caspase inhibitor blocks ischaemia-induced delayed neuronal death in the gerbil. Eur J Neurosci. 1998;10:777–81.
Ma J, Endres M, Moskowiz MA. Synergistic effects of caspase inhibitors and MK-801 in brain injury after transient focal cerebral ischemia in mice. Br J Pharmacol. 1998;124:756–62.
Men H, Agca Y, Riley LK, Critser JK. Improved survival of vitrified porcine embryos after partial delipidation through chemically stimulated lipolysis and inhibition of apoptosis. Theriogenology 2006;2008–16.
Bernardi P, Broekemeier KM, Pfeiffer DR. Recent progress on regulation of the mitochondrial permeability transition pore: a cyclosporing-sensitive pore in inner mitochondrial membrane. J Bioenerg Biomembr. 1994;26:509–17.
Xiong Y, Connolly T, Futcher B, Beach D. Human D-type cyclin. Cell. 1991;65:691–9.
Liu Y, Marraccino RL, Keng PC. Requirement for proliferating cell nuclear antigen expression during stages of the Chinese hamster ovary cell cycle. Biochemistry. 1989;28:2967–74.
Acknowledgments
Appreciation is extended to Dr Bin Cai for his review and comment and Xiao-Hua Hu from department of pathology for supporting this work.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Caspase inhibition is a valid therapeutic strategy in cryopreservation of ovarian tissue.
Rights and permissions
About this article
Cite this article
Zhang, JM., Li, LX., Yang, YX. et al. Is caspase inhibition a valid therapeutic strategy in cryopreservation of ovarian tissue?. J Assist Reprod Genet 26, 415–420 (2009). https://doi.org/10.1007/s10815-009-9331-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-009-9331-9