Abstract
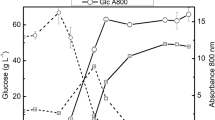
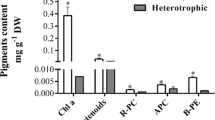
New food sources are urgently needed due to population growth. The production of microalgae biomass with high protein content is particularly of interest. Galdieria sulphuraria is an extremophilic red microalgae that can grow in acidic environments (pH 0 to 4) and above 40 °C. The aim of this work was to study the photoautotrophic growth and the biochemical composition of five G. sulphuraria strains to potentially produce single-cell protein. A kinetic study of cell growth and macromolecule content was performed in batch mode under controlled conditions: 42 °C, pH 2, constant illumination at 100 μmol photons m−2 s−1, 150 rpm, 0.5 vvm, and atmospheric CO2 (0.04%). The G. sulphuraria CCMEE 5587.1 reached 2.33 g L−1 of dry cell weight (DCW) (~ 9 × 107 cells mL−1) in 20 days, i.e., a productivity of ~ 110 mgDCW L−1 day−1 was achieved. The biomass from this strain shows a high protein content (~ 44% w/wDCW), 42.7% of essential amino acids, 4.7% (w/wDCW) of phycocyanin, 5.9% (w/wDCW) total carbohydrates, and 14.1% (w/wDCW) total lipids. A productivity of 21 t ha−1 year−1 could be attained assuming a straightforward scale-up in open ponds and reaching half the protein productivities obtained in the laboratory. This biomass composition is suitable for food purposes.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abd El-Hack ME, Abdelnour S, Alagawany M, Abdo M, Sakr MA, Khafaga AF, Mahgoub SA, Elnesr SS, Gebriel MG (2019) Microalgae in modern cancer therapy: current knowledge. Biomed Pharmacother 111:42–50
Ajayan KV, Selvaraju M, Thirugnanamoorthy K (2012) Enrichment of chlorophyll and phycobiliproteins in Spirulina platensis by the use of reflector light and nitrogen sources: an in-vitro study. Biomass Bioenerg 47:436–441
Assunção MFG, Varejão JMTB, Santos LMA (2017) Nutritional characterization of the microalga Ruttnera lamellosa compared to Porphyridium purpureum. Algal Res 26:8–14
Bajpai R, Zappi M, Dufreche S, Subramaniam R, Prokop A (2014) Status of algae as vehicles for commercial production of fuels and chemicals. In: Bajpai R, Prokop A, Zappi M (eds) Algal Biorefineries, vol 1. Cultivation of Cells and Products. Springer, Dordrecht, pp 3–24
Barbarino E, Lourenço SO (2005) An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J Appl Phycol 17:447–460
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210
Ben Hlima H, Dammak M, Karkouch N et al (2019) Optimal cultivation towards enhanced biomass and floridean starch production by Porphyridium marinum. Int J Biol Macromol 129:152–161
Benedetti M, Vecchi V, Barera S, Dall’Osto L (2018) Biomass from microalgae: the potential of domestication towards sustainable biofactories. Microb Cell Fact 17:173
Bishop W, Zubeck H (2012) Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J Nutr Food Sci 02:
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Brányiková I, Maršálková B, Doucha J, Brányik T, Bišova K, Zachleder V, Vítova M (2011) Microalgae-novel highly efficient starch producers. Biotechnol Bioeng 108:766–776
Bromke MA (2013) Amino acid biosynthesis pathways in diatoms. Metabolites 3:294–311
Chandra P, Sharma RK, Arora DS (2020) Antioxidant compounds from microbial sources: a review. Food Res Int 129:108849
Chronakis IS, Madsen M (2011) Algal proteins. In: Phillips GO, Williams PA (eds) Handbook of Food Proteins. Woodhead Publishing, London, pp 353–394
Embrapa (2021) Soja em números (safra 2019/20). Empresa Brasileira de Pesquisa Agropecuária. https://www.embrapa.br/soja/cultivos/soja1/dados-economicos. Accessed 2 June 2021
Ford TW (1979) Ribulose 1,5-bisphosphate carboxylase from the thermophilic, acidophilic alga, Cyanidium caldarium (Geitler): purification, characterisation and thermostability of the enzyme. Biochim Biophys Acta - Enzymol 569:239–248
Gaignard C, Gargouch N, Dubessay P, Delattre C, Pierre G, Laroche C, Fendri I, Abdelkaf S, Michaud P (2019) New horizons in culture and valorization of red microalgae. Biotechnol Adv 37:193–222
García JL, de Vicente M, Galán B (2017) Microalgae, old sustainable food and fashion nutraceuticals. Microb Biotechnol 10:1017–1024
García-Garibay M, Gómez-Ruiz L, Cruz-Guerrero AE, Bárzana E (2014) Single cell protein Yeasts and Bacteria. Encycl Food Microbiol 431–438
Garibay-Hernández A, Vazquez-Duhalt R, Serrano-Carreón L, Martinez A (2013) Nitrogen limitation in Neochloris oleoabundans: a reassessment of its effect on cell growth and biochemical composition. Appl Biochem Biotechnol 171:1775–1791
Graziani G, Schiavo S, Nicolai MA, Buono S, Fogliano V, Pinto G, Pollio A (2013) Microalgae as human food: chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria. Food Funct 4:144–152
Gross W, Schnarrenberger C (1995) Heterotrophic growth of two strains of the acido-thermophilic red alga Galdieria sulphuraria. Plant Cell Physiol 36:633–638
Henderson JW, Ricker RD, Bidlingmeyer BA, Woodward C (2000) Rapid, accurate, sensitive, and reproducible HPLC analysis of amino acids. Agilent Technologies. Application note No. 5980–1193E
Henkanatte-Gedera SM, Selvaratnam T, Karbakhshravari M, Myint M, Nirmalakhandan N, Van Voorhies W, Lammers PJ (2017) Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: laboratory to field scale demonstration. Algal Res 24:450–456
Koller M, Muhr A, Braunegg G (2014) Microalgae as versatile cellular factories for valued products. Algal Res 6:52–63
Koyande AK, Chew KW, Rambabu K, Tao Y, Chu DT, Show PL (2019) Microalgae: a potential alternative to health supplementation for humans. Food Sci Hum Wellness 8:16–24
Kursar TA, Alberte RS (1983) Photosynthetic unit organization in a red alga : relationships between light-harvesting pigments and reaction centers. Plant Physiol 72:409–414
Lafarga T, Fernández-Sevilla JM, González-López C, Acién-Fernández FG (2020) Spirulina for the food and functional food industries. Food Res Int 137:109356
Lima LMB, Lara LJC, Baiao NC, Cancado SD, Michell BC, Ferreira FC (2008) Effect of energy, lysine and methionine and cystine levels on performance and carcass yield of broiler chickens. Braz J Anim Sci 37:1424–1432
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Malavasi V, Soru S, Cao G (2020) Extremophile microalgae: the potential for biotechnological application. J Phycol 56:559–573
Manirafasha E, Murwanashyaka T, Ndikubwimana T, Ahmed NR, Liu J, Lu Y, Zeng X, Ling X, Jing K (2018) Enhancement of cell growth and phycocyanin production in Arthrospira (Spirulina) platensis by metabolic stress and nitrate fed-batch. Bioresour Technol 255:293–301
Maroneze MM, Siqueira SF, Vendruscolo RG, Wagner R, Ragagninde-de-Menezes C, Queiroz-Zepka L, Jacob-Lopes E (2016) The role of photoperiods on photobioreactors – a potential strategy to reduce costs. Bioresour Technol 219:493–499
Martinez-Garcia M, van der Maarel MJEC (2016) Floridoside production by the red microalga Galdieria sulphuraria under different conditions of growth and osmotic stress. AMB Express 6:71
Martinez-Garcia M, Stuart MCA, van der Maarel MJE (2016) Characterization of the highly branched glycogen from the thermoacidophilic red microalga Galdieria sulphuraria and comparison with other glycogens. Int J Biol Macromol 89:12–18
Martinez-Garcia M, Kormpa A, van der Maarel MJEC (2017) The glycogen of Galdieria sulphuraria as alternative to starch for the production of slowly digestible and resistant glucose polymers. Carbohydr Polym 169:75–82
Masojídek J, Torzillo G, Koblížek M (2007) Photosynthesis in microalgae. In: Richmond A, Hu Q (eds) Handbook of Microalgal Culture. John Wiley Blackwell, Oxford, pp 20–39
Moon M, Mishra SK, Kim CW, Suh WI, Park MS, Yang JW (2014) Isolation and characterization of thermostable phycocyanin from Galdieria sulphuraria. Korean J Chem Eng 31:490–495
Munasinghe-Arachchige SP, Delanka-Pedige HMK, Henkanatte-Gedera SM, Tchinda D, Zhang Y, Nirmalakhandan NM (2019) Factors contributing to bacteria inactivation in the Galdieria sulphuraria-based wastewater treatment system. Algal Res 38:101392
Najafpour GD (2007) Single-cell protein. In: Najafpour GD (ed) Biochem Eng Biotechnol. Elsevier Science, New York, pp 332–341
Nalage DN, Khedkar GD, Kalyankar AD, Sarkate AP (2016) Single cell proteins. Encycl Food Heal 790–794
Oesterhelt C, Schmälzlin E, Schmitt JM, Lokstein H (2007) Regulation of photosynthesis in the unicellular acidophilic red alga Galdieria sulphuraria. Plant J 51:500–511
Pade N, Linka N, Ruth W, Weber APM, Hagemann M (2015) Floridoside and isofloridoside are synthesized by trehalose 6-phosphate synthase-like enzymes in the red alga Galdieria sulphuraria. New Phytol 205:1227–1238
Price CA (1965) A membrane method for determination of total protein in dilute algal suspensions. Anal Biochem 12:213–218
Ritchie RJ (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89:27–41
Sakurai T, Aoki M, Ju X, Ueda T, Nakamura Y, Fujiwara S, Umemura T, Tsuzuki M, Minoda A (2016) Profiling of lipid and glycogen accumulations under different growth conditions in the sulfothermophilic red alga Galdieria sulphuraria. Bioresour Technol 200:861–866
Salbitani G, Carfagna S (2020) Different behaviour between autotrophic and heterotrophic Galdieria sulphuraria (Rhodophyta) cells to nitrogen starvation and restoration. Impact on pigment and free amino acid contents. Int J Plant Biol 11:7–11
Sheath RG, Vis ML (2015) Red Algae. In: Wehr JD, Sheath RG, Kociolek JP (eds) Freshwater algae of North America, 2nd edn. Academic Press, Boston, pp 237–264
Slocombe SP, Ross M, Thomas N, McNeill S, Stanley MS (2013) A rapid and general method for measurement of protein in micro-algal biomass. Bioresour Technol 129:51–57
Tibbetts SM, Milley JE, Lall SP (2015) Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J Appl Phycol 27:1109–1119
Vanthoor-Koopmans M, Wijffels RH, Barbosa MJ, Eppink MHM (2013) Biorefinery of microalgae for food and fuel. Bioresour Technol 135:142–149
Varshney P, Mikulic P, Vonshak A, Beardall J, Wangikara PP (2015) Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour Technol 184:363–372
Wan M, Wang Z, Zhang Z, Wang J, Li S, Yu A, Li Y (2016) A novel paradigm for the high-efficient production of phycocyanin from Galdieria sulphuraria. Bioresour Technol 218:272–278
Xie Y, Jin Y, Zeng X, Chen J, Lu Y, Jing K (2015) Fed-batch strategy for enhancing cell growth and C-phycocyanin production of Arthrospira (Spirulina) platensis under phototrophic cultivation. Bioresour Technol 180:281–287
Acknowledgements
The authors thank Mario A. Caro-Bermudez, Juan Manuel Hurtado Ramírez, Roberto Pablo Rodríguez Bahena, and Leonardo Peña-Carranza for technical support. Algae strains were obtained from the University of Göttingen, Germany (SAG 107.79, SAG 108.79, and SAG 21.92); Algae Culture Collection of the University of Texas, USA (UTEX 2919); and the Microbial Collection of Extreme Environments of the University of Oregon, USA (CCMEE 5587.1). CAMH held a scholarship from CONACyT – Mexico (699699).
Funding
This work was supported by Universidad Nacional Autónoma de México (UNAM) – Dirección General de Asuntos del Personal Académico (DGAPA), Grant PAPIIT-DGAPA-UNAM IT201119.
Author information
Authors and Affiliations
Contributions
CAM-H and AM, conception and design of the study and manuscript drafting and editing. CAM-H, cultivation of microalgae, biochemical composition analyses, and data analysis. AM, project administration and funding acquisition. All the authors (CAM-H, FV-LP, GTH-C, and AM) participated in data analyses, discussion of results, and writing and revising the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Montenegro-Herrera, C.A., Vera-López Portillo, F., Hernández-Chávez, G.T. et al. Single-cell protein production potential with the extremophilic red microalgae Galdieria sulphuraria: growth and biochemical characterization. J Appl Phycol 34, 1341–1352 (2022). https://doi.org/10.1007/s10811-022-02733-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02733-y