Abstract
Intertidal algae have to cope with diurnally and seasonally fluctuating environmental factors such as salinity, temperature, dehydration, and light. In New Zealand, solar radiation, including the ultraviolet wavelengths, is also an important stress factor for such algae. Therefore, two native (Bostrychia arbuscula W.H.Harvey [Ceramiales], Champia novae-zelandiae (J.D.Hooker & Harvey) Harvey [Rhodymeniales]) and one introduced red algal taxon (Schizymenia spp. J. Agardh [Nemastomatales]) were investigated over 12 months in terms of stress metabolites which contribute to ultraviolet radiation (UVR) and salinity tolerance. Mycosporine-like amino acids (MAAs), which act as sunscreens, and organic osmolytes were qualitatively and quantitatively analyzed. Porphyra-334, shinorine, and palythine were the most dominant MAAs yet distributed differently among the species. B. arbuscula showed a correlation between photosynthetically active radiation (PAR)/UVR and slightly higher MAA concentrations in summer. In contrast, C. novae-zelandiae displayed the lowest level of MAAs in summer, and no correlation was found between MAA values and solar radiation. In Schizymenia spp., the highest MAA amounts were found in summer, and for most months, a correlation with PAR/UV radiation was visible. While digeneaside and sorbitol were the dominant organic osmolytes in B. arbuscula, floridoside occurred in C. novae-zelandiae and Schizymenia spp. Only B. arbuscula exhibited higher organic osmolyte concentrations in summer. In contrast, floridoside contents in C. novae-zelandiae and Schizymenia spp. were low and highly variable over the course of the seasons. Our data indicate that both native red algal species are well acclimated to the intertidal zone. For the introduced Schizymenia spp., a more narrow salinity tolerance can be assumed, while the high MAA values may explain its establishment in New Zealand.
Similar content being viewed by others
Introduction
The intertidal zone is influenced by changing and fluctuating environmental factors, such as radiation, temperature, salinity, and mechanical forces, resulting in numerous physicochemical gradients (Lüning 1990). Macroalgae living in this zone have to tolerate these diurnally and seasonally changing environmental conditions (Davison and Pearson 1996; Bischof et al. 2006; Diehl et al. 2019), because they often act as stressors causing vertical and biogeographical distribution patterns in intertidal macroalgal communities (Lüning 1990). In the intertidal zone, solar irradiance is such a stress factor for macroalgae (Bischof et al. 2006), and in particular, ultraviolet radiation (UVR) has strong damaging effects on algal cells, their metabolism and physiology (Hoffman et al. 2003; Hanelt et al. 2006; Hanelt and Figueroa 2012). UVR, especially UVB (280–315 nm), is absorbed by different biomolecules such as nucleic acids (Vass et al. 2005; Bischof et al. 2006; Karsten 2008). As a result, UVB has disturbing effects on algal photosynthesis (e.g., Hoffman et al. 2003; Hanelt et al. 2006; Hanelt and Figueroa 2012). For example, UVR leads to lower photosynthetic activity (Hanelt and Figueroa 2012), because of damage to the CO2-fixing enzyme Rubisco, the functional decoupling of LHCs from the photosystems, the photodestruction of pigments, or the inactivation of chloroplast ATPase (Holzinger et al. 2004; Bischof and Rautenberger 2012). Furthermore, UVB induces the formation of intracellular reactive oxygen species (ROS) and can cause ultrastructural changes in cells (Cheloni and Slaveykova 2018; Rangel et al. 2020). Ultrastructural changes include swollen mitochondrial cristae, detached phycobilisomes, and drastic changes in the arrangement of thylakoid membranes or even destroyed thylakoids and damage to the plasmalemma (Holzinger et al. 2004; Karsten 2008).
The most common biochemical acclimation of red algae against UVR is the biosynthesis and accumulation of mycosporine-like amino acids (MAAs) as UV-protective molecules (Carreto and Carignan 2011). MAAs are derivatives of amino acids which contain cyclohexanone or cyclohexenimine ring structures (Dunlap and Yamamoto 1995). MAAs are water-soluble and preferentially absorb UVR in the range from 310 to 360 nm (Nakamura et al. 1982; Dunlap and Yamamoto 1995; Karsten 2008). Until now, many different MAAs were identified, but the most prevalent UV-sunscreens in red algae are porphyra-334, shinorine, and palythine (Hoyer et al. 2002; Orfanoudaki et al. 2018, 2019). After Hoyer et al. (2001, 2002), red algal species can be classified into different response types, based on their MAA concentrations: type I—no MAAs, type II—MAAs inducible in variable concentrations, and type III—permanently high MAA concentrations. The formation and accumulation of MAAs in intertidal red algae is a species-specific process (Hoyer et al. 2001, 2002).
An additional important environmental stress factor for intertidal macroalgae is salinity change, which is influenced by tides, hydrological conditions, wind, precipitation and evaporation (Karsten 2012). Under hypo- or hypersaline conditions, macroalgae exhibit protective mechanisms such as osmotic acclimation (Kirst 1990; Karsten 2012). Based on the salinity changes macroalgae can tolerate, they are characterized as euryhaline or stenohaline (Russell 1987). Under salinity stress low-molecular-weight organic solutes are typically synthesized and accumulated (hypersaline conditions) or degraded (hyposaline conditions) (Ben-Amotz and Avron 1983). Some of those organic osmolytes (e.g. floridoside or mannitol) are identical with the main photosynthetic products of the algae under investigation (Karsten 2012). Additionally, organic osmolytes exert other biochemical functions as they can act as compatible solutes (protect enzymes and structural molecules against denaturation), i.e. these compounds are highly soluble in the cytoplasm and non-toxic at high concentrations (Kirst 1990; Shetty et al. 2019). Photosynthesis and respiration are strongly affected by salinity changes (Sudhir and Murthy 2004). High salinities typically inhibit at least three processes in the photosynthetic machinery: (1) photoactivation of electron flow on the reducing site of PSI, (2) electron flow at the water splitting site of PSII, and (3) transfer of light energy between pigment complexes (Kirst 1990). In the last decades, much progress was made in identifying low-molecular-weight carbohydrates (LMWCs), especially in red algae (Karsten 2012). Different organic osmolytes were identified in red algae such as mannitol, dulcitol, sorbitol, and trehalose (Karsten et al. 1996, 1997; Eggert and Karsten 2010), and these data were also used for chemotaxonomic purposes (Karsten et al. 1999). Recent studies showed a complex picture of the diversity and distribution of LMCWs for red algae and revealed strong group-specific patterns (Eggert et al. 2007; Karsten et al. 2007; Diehl et al. 2019). Most species of the Florideophyceae typically contain floridoside (Karsten et al. 2007). Species within the order Ceramiales synthesize digeneaside instead of floridoside, and some genera (e.g., Bostrychia and Caloglossa) also synthesize untypical polyols such as sorbitol, dulcitol, or even mannitol (Karsten et al. 2005, 2007). Especially in New Zealand, little is known about organic osmolytes in red algae with the exception for Pyropia plicata (Diehl et al. 2019).
New Zealand’s coastline has rich seaweed diversity, especially red macroalgae (Adams 1994; Hurd et al. 2004; Nelson 2013), but there exist only few ecophysiological studies on intertidal macroalgae, and only a few have been conducted on stress response patterns (Lamare et al. 2004; Schweikert et al. 2011; Muangmai et al. 2015; Bollen et al. 2016; Diehl et al. 2019). The consistent result of the previous studies is that intertidal red algae contain stress metabolites in high concentrations. In climate change scenarios, not only will UVB radiation potentially increase (McKenzie et al. 1999) but also nonindigenous marine species become common due to changing environments and continued ship traffic (Schaffelke et al. 2006). Abiotic and biotic factors are important for the success of introduced macroalgae in their new habitat (Alexander and Edwards 2010). Successful invasive species are often adapted to variable abiotic conditions (Bollen et al. 2016). In the last few years, many introduced species were identified based on molecular techniques in New Zealand (D’Archino et al. 2007; Nelson et al. 2013; D'Archino and Zuccarello 2014; D’Archino et al. 2015; Garbary et al. 2020). For example, Schizymenia apoda was first recorded in 2014 (D’Archino and Zuccarello 2014).
In the present study, stress metabolites of two native red algal species were followed over the course of the seasons and compared with those of introduced species. The native Bostrychia arbuscula (Ceramiales, Rhodomelaceae) is a widely distributed, tufted macroalga growing attached by haptera in the upper rocky intertidal (Nelson 2013). Bostrychia species are euryhaline with a broad salinity tolerance (Muangmai et al. 2015). Champia novae-zelandiae (Rhodymeniales, Champiaceae), another native taxon, is also widely distributed (Nelson 2013). Champia novae-zelandiae is found in the intertidal and upper subtidal, attached by discoidal holdfasts to rocks or epiphytically on large brown seaweeds (Nelson 2013). The introduced species belonged to the genus Schizymenia (Nemastomatales, Schizymeniaceae). Schizymenia apoda was first reported as an introduced species in New Zealand in Wellington Harbor. Recently, a new introduced Schizymenia species, S. dubyi, has been discovered in Wellington Harbor (D’Archino and Zuccarello 2020, and unpublished observations). Both these species are known to be introduced in various locations around the world (Gabriel et al. 2011; Ramirez et al. 2012; Saunders et al. 2015; Gunnarsson et al. 2020). The foliose Schizymenia spp. is mainly found in the low intertidal to shallow subtidal and in large tidal pools (Guiry and Guiry 2020).
The aim of this study was to qualitatively and quantitatively evaluate stress metabolite patterns over the course of the seasons in these native and introduced red algal taxa to better understand their tolerance against UVR and salinity fluctuations. It was hypothesized that both native species contain higher MAA concentrations compared with the introduced species, because of their different vertical position in the intertidal zone. For the introduced species, seasonally changing MAA values were assumed with highest MAA levels in summer. In addition, organic osmolyte concentrations were further hypothesized to follow the exposure gradient, i.e., being higher in the upper intertidal compared with lower amounts in the mid intertidal or upper subtidal with probably no effect of seasonality.
Material and methods
Species collections
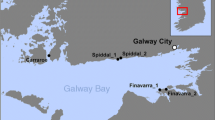
The native Bostrychia arbuscula and Champia novae-zelandiae were collected during low tide from Moa Point (41° 20′ 31.6″ S, 174° 48′ 35.4″ E) on the south coast of Wellington, New Zealand. Bostrychia arbuscula was collected on rocks in the high-intertidal facing south (Fig. 1b). Champia novae-zelandiae was found in a more sheltered mid-intertidal zone, often submerged with water (Fig. 1d). Schizymenia spp. was collected in the Wellington harbor at Whairepo Lagoon (41° 17′ 17.86″ S, 174° 46′ 47.26″ E) in large rock pools, always submerged with seawater (Fig. 1f). The two Schizymenia species (S. apoda and S. dubyi) are indistinguishable morphologically, so a molecular survey was done to identify the species in the Whairepo Lagoon (Supplementary Tab. S1). The molecular data indicate that both species co-occur in close to equal amounts in this location (unpublish. data). Additionally, the mycosporine-like amino acids (MAAs) and organic osmolytes were analyzed in both Schizymenia species separately, and the same MAAs and organic osmolytes were found (Suppl. Figs. S5–S6). Based on the similarity in the biochemical profiles and due to the taxonomic difficulty of separating both taxa, the data used in the present study is of combined Schizymenia spp.
Abiotic conditions
The abiotic data included global radiation (GR), ultraviolet radiation (UVR), sunshine hours, precipitation, and ozone which were provided by the New Zealand Institute for Water and Atmosphere (NIWA) and downloaded from the National Climate Database CliFlo (http://cliflo.nowa.co.nz/, accessed on 15 Jan 2020) (CliFlo, National Climate Database 2017). Photosynthetically active radiation (PAR) was derived from the global radiation data (Physiology University of Guelph 2008) and UVB radiation data were calculated from the UV Index (McKenzie et al. 2004). The seawater temperature was taken from the dataset of the Greater Wellington Regional Council (http://graphs.gw.govt.nz/) (Greater Wellington Region Council (GWRC) 2017). This monitoring station is located in the harbor of Wellington at Queens Wharf. For details, see Supplementary Table S2.
Seasonal sampling
All algae were collected once per month (Suppl. Tab. S3) and preserved by drying in an oven at 60 °C and stored in the dark until further analyses. The salinity at Moa Point (36 SA) and in the Whairepo Lagoon (approx. 34 SA) was measured when collecting, using a Pocket Refractometer (PAL-06S, Atago, USA).
Extraction and measurement of MAAs
A 1220 Infinity II HPLC system (Agilent Technologies, USA) with a diode array detector (DAD) was used to identify and quantify mycosporine-like amino acids (MAAs) according to Karsten et al. (2009). The first step of the MAA extraction was to add 1 mL of 25% aqueous methanol (v/v) to approx. 15 mg of dried algal tissue in a screw-capped vial followed by incubation in a water bath at 45 °C for 3–4 h. The samples were vortexed regularly to optimize extraction, and finally centrifuged at 6,390×g for 5 min (Biofuge pico, Heraeus, Germany). Eight hundred microliters of the supernatant was transferred into a new vial and evaporated overnight in a SpeedVac (RVC 2-25 CDplus, Christ, Germany). The dried pellet was re-dissolved in 800 μL of HPLC-water, vortexed for 30 s, and centrifuged (21,650×g, 5 min, Megafuge, Heraeus, Germany). The clear supernatant was transferred into a HPLC vial to analyze the MAAs. A Phenomenex Synergi 4μ fusion RP-column (250 × 3 mm I.D., Phenomenex, Germany) with a guard cartridge (Phenomenex, Fusion-RP 4 × 3 mm I.D., Germany) was used. The mobile phase (eluent) was 2.5% methanol plus 0.1 % acetic acid in water, with a flowrate of 0.5 mL min−1, at 150 bar pressure and room temperature (25 °C). The sample chromatograms were compared with those of the measured standards of palythine, shinorine, asterina-330, porphyra-334, and mycosporine-glycine to identify and quantify the algal MAAs. The identified peaks were manually integrated, and the area under each peak was used for calculations of the MAA concentration in mg per g dry weight (mg g−1 DW).
Extraction and measurement of organic osmolytes
A 1260 Infinity HPLC system (Agilent Technologies, USA) with a refractive index (RI) detector was used to qualitatively and quantitatively determine organic osmolytes according to Diehl et al. (2019). For the extraction of the organic osmolytes, again about 15 mg dried tissue and 1 mL of 70% aqueous ethanol (v/v) in a screw-capped vial were placed in a water bath at 70 °C for 3–4 h. The vials were vortexed regularly and finally centrifuged at 6390×g for 5 min (Biofuge pico, Heraeus, Germany). Then, 800 μL of the supernatant was transferred into a new vial and evaporated overnight in the SpeedVac. On the next day, 800 μL of HPLC-water was added to the dried pellet, vortexed for 30 s, and treated in an ultrasonic bath for about 10 min to re-dissolve the pellet completely. Finally, the vials were again centrifuged for 5 min (21,650×g), and the supernatants transferred into HPLC vials. Two different analytical methods were applied to identify the organic osmolytes. A Phenomenex Rezex ROA-Organic Acid column (300 × 7.8 mm, 8 μm, Phenomenex, Germany), protected by a guard cartridge (Phenomenex, Carbo-H 4 × 3 mm I.D.), was used to identify and analyze heterosides. The mobile phase (eluent) consisted of 5 mM aqueous sulfuric acid run at a flowrate of 0.4 mL min−1 with 21 bar pressure and with 70 °C as the column temperature. The injection volume was 20 μL. A BIO-RAD Aminex Fast Carbohydrate Analysis Column HPAP (100 × 7.8 mm, 9 μm, BIO-RAD, USA) was used together with a guard cartridge (Phenomenex, Carbo-H 4 × 3 mm I.D.) to identify polyols like sorbitol. The eluent was pure HPLC water with a flowrate of 1 mL min−1, 35 bar pressure, and 70 °C as column temperature. The injection volume was 10 μL. For the standards, sorbitol and digeneaside, a 10-point calibration curve was applied with the lowest concentration of 1 mM up to the highest concentration of 10 mM. For floridoside, only a 6-point calibration curve was used with concentrations from 0.5 mM up to 3 mM. The peaks in the sample chromatograms were identified by retention time. All peaks were integrated, and absolute concentrations were calculated in mmol per kg dry weight (mmol kg−1 DW) by determining the integrated peak area and the slope of the calibration line.
Statistics
All statistical analyses were performed using SPSS Statistics 25 (IBM, USA). To test for significant differences of organic osmolyte and MAA contents between the sampling time points, a one-way ANOVA was performed. The assumptions were checked (Normality with the Shapiro-Wilk Test and Q-Q-Plots, outliers with a boxplot, homogeneity of variances with Levene’s test (p > 0.05)). The normality of the data was not found in all cases, but this violation was neglected due to the small sample and replicate sizes (Underwood 1997). If the ANOVA or Welch ANOVA (for violation of assumptions, if Levene’s test was significant) showed significant differences (p < 0.05), a post hoc Tukey’s HSD or a Games-Howell post hoc test (for Welch-ANOVA) was used for more detailed interpretation and to create the case lettering of the graphs for visualization of the significant differences of the MAA and organic osmolyte levels between the different months. For all ANOVAS, “months” was used as the independent variable (= level), and “MAA” and “organic osmolyte concentration” were used as the dependent variables. For detailed results of the statistical analysis, see Supplementary Table S4–S9.
Results
Abiotic conditions between July 2018 and August 2019
The abiotic data for Wellington showed strong seasonal changes over the course of the year (Suppl. Figs. S1–S2). The mean daily global radiation, the mean daily PAR, and also the mean daily ultraviolet B (UVB) radiation were highest in Wellington summer (December–February) and decreased continuously toward winter. The ultraviolet index (UVI) showed a similar course over the seasons like the UVB radiation. Ozone concentrations varied slightly over the course of the year between 260 and 360 Dobson units (DU). The total sunshine hours per month varied annually between approx. 100 h in southern winter and 260 h in southern summer. The total precipitation (rainfall) per month varied strongly during the year (Suppl. Fig. S3), with lowest values in January and February 2019 (< 25 mm) and highest amounts in April and July 2019 with over 200 mm. The water temperature in Wellington ranged between 10°C in winter up to 20°C in summer (data not shown).
MAA levels over the course of the year (July 2018–August 219)
In B. arbuscula, four different MAAs were identified: porphyra-334, palythine, asterina-330, and shinorine. Porphyra-334 and palythine were the two quantitatively dominant MAAs (Fig. 2). Asterina-330 and shinorine were only found in traces (less than 0.1 mg g−1 DW). Due to non-homogeneous data, only the total MAA concentrations were statistically analyzed. The total MAA contents (poryhra-334 and palythine) varied over the course of the year between July 2018 and August 2019 and were significantly different between the months (ANOVA: F(11, 24) = 4.178, p < 0.005; Suppl. Tab. 4). The highest total MAA amount was measured in summer (January 2019) with 8.2 ± 1.5 mg g−1 DW. The lowest total MAA concentrations were found in winter (July: 5.2 ± 0.3 mg g−1 DW and August: 5.1 ± 0.7 mg g−1 DW). For all months, porphyra-334 made up the largest fraction of the total MAA concentration and proportionally changed more than palythine between the months. The palythine values ranged between 0.6 and 1.5 mg g−1 DW (Fig. 2). The highest palythine concentration was detected in December 2018, and the lowest amount was found in August 2019. The porphyra-334 concentration varied from 4 to 7 mg g−1 DW over the months and was highest in January 2019 (7.1 ± 1.2 mg g−1 DW) and lowest in November 2018 (3.9 ± 1.2 mg g−1 DW). A positive correlation of UVR (UVB and UVI) and PAR with the total MAA concentration of B. arbuscula was recognizable, i.e., the higher the radiation, the more MAAs were synthesized and accumulated (Fig. 2).
Monthly mycosporine-like amino acid (MAA) concentrations of Bostrychia arbuscula over the course of the seasons (July 2018 to August 2019) expressed as mean values ± SD (n = 3). n.a. no samples available. The left y-axis shows the two dominant MAA concentrations of porphyra-334 and palythine (mg g−1 DW). The mean daily UVB radiation (W m−2) and the mean daily UV Index (UVI) are also shown along with the mean daily photosynthetically active radiation (PAR [μmol photons m−2 s−1]). Abiotic data were downloaded from CliFlo (2020). Lowercase letters indicate significant differences in the total MAA concentration between the different months (p < 0.05)
In addition, three unidentified MAAs were found in B. arbuscula: MAA_1 (ʎ ~ 331 nm, retention time 3.6 min), MAA_2 (ʎ ~ 356 nm, retention time 9.8 min) and MAA_3 (ʎ ~ 361 nm, retention time 10.1 min). These MAAs were not analyzed quantitatively as they contributed < 20% to the total MAA concentration (Fig. 3).
Percentages of all mycosporine-like amino acids (MAAs) of the total MAA content of Bostrychia arbuscula, collected over the course of the seasons (July 2018 to August 2019) at Moa Point, Wellington, New Zealand; n.a. no samples collected. The y-axis shows the integrated area in % for each MAA, measured with high performance liquid chromatography (HPLC)
In C. novae-zelandiae, two MAAs were found: palythine and shinorine (Fig. 4). Due to non-homogeneous data and for comparison with the other algae, only the total MAA concentrations were statistically analyzed. The total MAA contents varied over the course of the year from July 2018 to August 2019 and were significantly different between the months (ANOVA: F(11,24) = 5.25, p < 0.005; Suppl. Tabl. 5). The highest total MAA amounts were detected in winter months: July 2018 (7.8 ± 0.9 mg g−1 DW), September 2018 (8.1 ± 0.9 mg g−1 DW) and May 2019 (8.3 ± 1.2 mg g−1 DW). These 3 months are also significantly different to the lowest MAA values found in summer (November 2018, December 2018, and January 2019; Fig. 4). The lowest total MAA concentration was measured in December 2018 (3.4 ± 1.1 mg g−1 DW). The concentrations of both the MAAs palythine and shinorine changed similarly over the course of the seasons. The palythine concentration varied between 1.9 and 5.4 mg g−1 DW, while the shinorine content ranged between 1.1 and 3.7 mg g−1 DW. In most of the months, the palythine level made up the larger proportion of the total MAA amount compared with shinorine. One exception is July 2019 where both MAA levels were nearly identical (Fig. 4). Overall, the total MAA concentrations were lowest in summer and higher in winter, especially in September 2018 and May 2019. No correlation between various radiation parameters and the total MAA concentration could be identified in C. novae-zelandiae (Fig. 4).
Monthly mycosporine-like amino acid (MAA) concentrations of Champia novae-zelandiae over the course of the seasons (July 2018 to August 2019) expressed as mean values ± SD (n = 3); n.a. no samples available. The left y-axis shows the two MAA concentrations of palythine and shinorine [mg g−1 DW]. The mean daily UVB radiation [W m−2] and the daily UV Index (UVI) are also shown along with the mean daily photosynthetically active radiation (PAR [μmol photons m−2 s−1]). Abiotic data were downloaded from CliFlo (2020). Lowercase letters indicate significant differences in the total MAA concentration between the different months (p < 0.05)
Shinorine was the quantitatively dominant MAA in Schizymenia spp., and palythine was only found in trace concentrations in each month (< 1 mg). Therefore, the palythine concentrations were not further analyzed statistically and are not included in Fig. 4. The shinorine concentration varied strongly over the course of the year and was significantly different between the months (Welch ANOVA: F(9,8.08) = 22.21, p < 0.005; Suppl. Tab. 6). The highest shinorine values were found in December 2018 (9.6 ± 0.7 mg g−1 DW), June 2019 (9.4 ± 0.8 mg g−1 DW), and October 2018 (7.1 ± 0.5 mg g−1 DW. The two highest shinorine concentrations (December 2018 and June 2019) were not significantly different to the shinorine concentration in October 2018 but significantly different to all other months: Fig. 5). The lowest shinorine amount was detected in September 2018 (3.1 ± 0.3 mg g−1 DW). Overall, the shinorine concentrations increased toward summer and decreased toward winter with one exception in June (winter) 2019, where the high shinorine value exceeded the months before and afterward. A positive correlation of the radiation parameters (UVB, UVI, PAR) with the shinorine concentration of Schizymenia spp. was recognizable for most of the months (Fig. 5).
Monthly mycosporine-like amino acid (MAA) concentrations of Schizymenia spp. over the course of the seasons (July 2018 to August 2019) expressed as mean values ± SD (n = 3); n.a. no samples available. The left y-axis shows the shinorine concentrations [mg g−1 DW]. The mean daily UVB radiation [W m−2] and the daily UV Index (UVI) are also shown along with the mean daily photosynthetically active radiation (PAR [μmol photons m−2 s−1]). Abiotic data were downloaded from CliFlo (2020). Lowercase letters indicate significant differences in the total MAA concentration between the different months (p < 0.05)
Organic osmolytes over the course of the year (July 2018–August 2019)
Bostrychia arbuscula contained digeneaside and sorbitol as organic osmolytes (Fig. 6). The total concentration of both organic osmolytes varied over the course of the year and showed significant differences between the months (ANOVA: digenaside F(11,24) = 4.03 p < 0.005, sorbitol F(11,24) = 25.37, p < 0.005; Suppl. Tab. 7). The digeneaside values did not vary greatly between the months and ranged between 100 and 166 mmol kg−1 DW, with the highest amount in December 2018 (Fig. 6). In contrast, the sorbitol concentrations exhibited a wide range between the different months from 54 to 482 mmol kg−1 DW. The highest sorbitol levels were found in summer (December 2018: 481.6 ± 22.2 mmol kg−1 DW and January 2019: 401.3 ± 47.2 mmol kg−1 DW). The lowest concentration of sorbitol was measured in winter in July 2018 (54.4 ± 31.9 mmol kg−1 DW) which was similar to the sorbitol concentrations in May, June, July, and August 2019 and significantly different to the remaining months (Fig. 6). Overall, the sorbitol amounts increased over spring toward summer and decreased over autumn toward winter. A correlation with sunshine hours and rainfall was only recognizable for the sorbitol concentrations in B. arbuscula. Sorbitol contents followed the same seasonal course as sunshine hours and rainfall (Fig. 6).
Monthly organic osmolyte concentrations of Bostrychia arbuscula, collected at Moa Point, Wellington, New Zealand, between July 2018 to August 2019, and expressed as mean values ± SD (mmol kg-1 DW, n = 3); n.a. no samples collected. The left y-axis shows the organic osmolyte concentrations of sorbitol and digeneaside. Sunshine hours (h) and total rainfall (mm) are also shown. Abiotic data were downloaded from CliFlo (2020). Lowercase letters indicate significant differences in the digeneaside concentrations between the different months and capital letters indicate significant differences in the sorbitol concentrations between the different months (p < 0.05)
Champia novae-zelandiae contains only one organic osmolyte, floridoside (Fig. 7). The floridoside concentrations of C. novae-zelandiae were significantly different between the months, ranging from 105 to 243 mmol kg−1 DW (Welch ANOVA: F(11,9.38) = 15.16, p < 0.005; Suppl. Tabl. 8). The highest floridoside values were found in November 2018 (243.3 ± 15.3 mmol kg−1 DW) and September 2018 (233.9 ± 7.4 mmol kg−1 DW). In these months, the floridoside contents were significantly different to July 2018, April 2019, and June and July 2019 (Fig. 7). The lowest floridoside values were measured in April 2019 (106.7 ± 20.5 mmol kg−1 DW) and June 2019 (104.8 ± 17.9 mmol kg−1 DW). For most of the months, there were no significant differences in the floridoside concentrations (Fig. 7). Over winter and spring (July to November 2018), the floridoside amounts increased and then decreased over summer until April 2019. In autumn, the floridoside concentrations fluctuated and did not show a clear trend. In July and August 2019, the floridoside value started to increase again. Except for the summer months, the sunshine hours and rainfall correlate with the floridoside concentrations of C. novae-zelandiae (Fig. 7).
Monthly organic osmolyte concentrations of Champia novae-zelandiae, collected at Moa Point, Wellington, New Zealand, between July 2018 and August 2019, and expressed as mean values ± SD (mmol kg−1 DW, n = 3); n.a. no samples collected. The left y-axis shows the organic osmolyte concentration of floridoside. Sunshine hours (h) and total rainfall (mm) are also shown. Abiotic data were downloaded from CliFlo (2020). Lowercase letters indicate significant differences in the floridoside concentrations between the different months (p < 0.05)
Schizymenia spp. contained floridoside as the main organic osmolyte (Fig. 8). An additional compound was detected with the HPLC but could not be chemically identified. Hence, the unknown compound was not quantitatively analyzed over the course of the year (Supplementary Fig. S5). The percentage of the unknown compound of the total produced organic osmolyte compounds of Schizymenia spp. varied between 22 and 34% (Supplementary Fig. S5). The concentrations of floridoside varied greatly between months, ranging from 64 to 145 mmol kg−1 DW, and the levels were significantly different between the months (Welch ANOVA: F(9,7.9) = 49.11, p < 0.005; Suppl. Tab. 9). The highest floridoside contents were found in October 2018 (145.0 ± 9.5 mmol kg−1 DW) and August 2019 (138.3 ± 8.3 mmol kg−1 DW). These two floridoside concentrations were significantly different to all other months. The lowest floridoside values were measured in August 2018 (68.4 ± 1.3 mmol kg−1 DW) and May 2019 (64.4 ± 12.9 mmol kg−1 DW). The floridoside concentrations of December 2018 and July 2019 were significantly different to the two highest amounts in October 2018 and August 2019 and to the lowest floridoside level in May 2019 (Fig. 8).
Monthly organic osmolyte concentrations of Schizymenia spp., collected at Whairepo Lagoon, Wellington, New Zealand, between July 2018 and August 2019, and expressed as mean values ± SD (mmol kg−1 DW, n = 3); n.a. no samples collected. The left y-axis shows the organic osmolyte concentration of floridoside. Sunshine hours (h) and total rainfall (mm) are also shown. Abiotic data were downloaded from CliFlo (2020). Lowercase letters indicate significant differences in the floridoside concentrations between the different months (p < 0.05)
Over spring (August to October 2018), the floridoside values increased and then decreased into summer and autumn until May 2019, when they started to increase again (Fig. 8). Except for the summer months and the winter months in 2019, the sunshine hours and rainfall positively correlate with the floridoside concentrations of Schizymenia spp. (Fig. 8).
Discussion
Our results showed that Bostrychia arbuscula contained a larger number of different MAAs in contrast to Champia novae-zelandiae and Schizymenia spp., which had only two different MAAs. Additionally, the MAA levels were variable over the seasons between the algae and the seasonal patterns were different.
The results for the organic osmolytes over the course of the year revealed that B. arbuscula contained digeneaside and sorbitol, C. novae-zelandiae had only floridoside, and in Schizymenia spp., an unknown compound was found in addition to floridoside. For B. arbuscula and Schizymenia spp., high variability between the months was found in the concentrations of the total organic osmolytes, while in C. novae-zelandiae, the floridoside levels did not vary much between the months.
Abiotic conditions between July 2018 and August 2019
The typical meteorological conditions in Wellington changed over the course of the field sampling between winter 2018 and winter 2019. The winter and spring 2018 started with rainfall patterns almost identical to the monthly averages from 1981 to 2010 (NIWA 2020). However, at the beginning of 2019, an El Niño event in the central Pacific (which lasted until July 2019) provided sunnier and drier weather for the whole of New Zealand (NIWA 2020). As a result, the rainfall in Wellington was below average in January and February 2019, and reversely above average in March to May. The dry and sunny summer of 2018/19 in Wellington was also reflected in more hours of sunshine compared with previous periods.
The UV index (UVI) is a useful parameter for solar radiation intensity, as it includes all factors affecting incident UVR such as cloud coverage, season, altitude, air pollution, and surface reflections (McKenzie et al. 2004; NIWA 2016). The UVI in New Zealand is about 40% higher compared with similar latitudes in the northern hemisphere due to differences in atmospheric ozone concentration, sun-earth separation, and air pollution. Therefore, UVR could be a strong stressor for intertidal algae in New Zealand.
MAAs over the course of the seasons
Although B. arbuscula showed the highest MAA concentrations in summer when UVR was enhanced, the variation of total MAA values over the course of seasons was rather small. Based on these findings, B. arbuscula can be classified as MAA response type III according to Hoyer et al. (2001). Permanently high MAA contents are typical for upper intertidal red algae like Porphyra (Pyropia) spp. or Bangia spp. (Hoyer et al. 2001, 2002). Diehl et al. (2019) found also consistently high MAA concentrations in Pyropia plicata from New Zealand and classified this species as a type III responder too. Bostrychia arbuscula and P. plicata grow close to each other in the upper intertidal zone at Moa Point, Wellington, New Zealand, and both species exhibited similar total MAA values (Diehl et al. 2019). However, both taxa differed in the seasonal response patterns, as for P. plicata, the highest MAA amounts were measured in winter and lowest in summer (Diehl et al. 2019), which is the opposite pattern to B. arbuscula, pointing to species-specific responses.
Compared with B. arbuscula, C. novae-zelandiae showed a different MAA pattern over the course of the seasons. The total MAA contents were twofold higher in spring and autumn then in summer, when UVR/UVI was enhanced. Champia novae-zelandiae preferentially grows in the lower intertidal zone and hence might be considered as MAA response type II due to its variable MAA amounts (Hoyer et al. 2001). Type II contains mainly intertidal red algae with inducible MAA concentrations (Hoyer et al. 2001). This could support the assumption that besides UVR, other environmental factors might influence the induction of MAAs, and this needs to be examined in future studies.
The introduced species Schizymenia spp. showed a seasonal trend in the MAA concentrations with increasing values toward summer and decreasing levels in autumn and winter. As the MAA content in Schizymenia spp. is also strongly varying, this species can also be assigned to MAA response type II (Hoyer et al. 2001). The rock pool where Schizymenia spp. was collected differs from Moa Point as a habitat, as there is much less wave movement and water mixture, resulting in higher water temperatures and different nutrient conditions, which might influence the MAA concentrations (Karsten 2008).
Compared with other red algal species, the MAA concentrations determined in the present study, with up to 8–9 mg total MAAs g−1 DW, must be considered as high and is on average by 50 to 100% enhanced compared to red algae from Helgoland (German Bight), Spain, or the polar regions (Karsten et al. 1998a, b; Hoyer et al. 2001).
Comparison of MAAs between the species
A more detailed comparison of the number of individual MAAs in all three red algae demonstrated considerable differences with B. arbuscula exhibiting more sunscreen compounds than C. novae-zelandiae and Schizymenia spp. We only identified two MAAs in C. novae-zelandiae and Schizyemnia spp. with the used HPLC method. In contrast, Orfanoudaki et al. (2019) showed that both species collected from the same location contained more MAAs (Schizymenia spp.: 2 additional MAAs, Champia novae-zelandiae: 5 additional MAAs), by using a more sensitive LC-MS (liquid chromatography-mass spectrometry) method. In B. arbuscula, porphyra-334 and palythine were identified together with an unknown MAA (MAA_1), which were the most dominant sunscreen compounds. Furthermore, shinorine and asterina-330 were found in small amounts. MAA_1 (ʎ ~ 331 nm, RT: ~ 3.57 min) might be palythinol or aplysiapalythin based on the paper of Orfanoudaki et al. (2019). Two other unknown MAAs (MAA_2: ʎ ~ 356 nm, RT: ~ 9.81 min) and MAA_3 (ʎ ~ 361 nm, RT: ~ 10.14 min) were detected in some monthly samples of B. arbuscula. MAA_2 and MAA_3 are not discussed further, and it can be assumed that these are partially degraded or transformed MAAs, supported by the study of Orfanoudaki et al. (2019) in which these compounds were not found. It seems that the genus Bostrychia is in general chemically very rich in synthesizing diverse MAAs, and this can be explained by its specific mainly exposed habitats such as the upper intertidal zone on rocks or as epiphytes on mangrove roots (Orfanoudaki et al. 2019), where strong insolation and salinity fluctuations are key factors.
In C. novae-zelandiae and Schizymenia spp., only palythine and shinorine were found. While in C. novae-zelandiae palythine and shinorine are both quite abundant over the course of the year, in Schizymenia spp., only shinorine was the quantitatively dominant MAA. These data indicate that the MAA inventory and its regulation over the course of the seasons is species-specific as supported by the few other studies on red algae from New Zealand (Lamare et al. 2004; Diehl et al. 2019), as well as by the fundamental investigations of Hoyer et al. (2001, 2002) on numerous red algal taxa from Antarctica. The latter authors reported some taxa not capable to synthesize MAAs at all (deep water species) as well as different MAA response types in relation to controlled PAR, PAR + UVA, and PAR + UVA/B conditions. As no consistent MAA induction patterns could be found, even for individual MAAs, Hoyer et al. (2002) concluded that induction, formation, and accumulation of individual MAAs is a very flexible and species-specific mechanism. Besides UVR and PAR, also other abiotic factors might influence algal MAA concentrations. Carreto and Carignan (2011) have identified dehydration, thermal stress, or nutrient availability (especially nitrogen) as key factors influencing MAA biosynthesis. Ammonium supply stimulated the accumulation of MAAs in two Porphyra species (Korbee et al. 2005). It can therefore be assumed that an interaction of different environmental factors would be responsible for the seasonal accumulation patterns of MAAs in red algae of New Zealand.
Organic osmolytes over the course of the seasons
Bostrychia arbuscula synthesized the two organic osmolytes digeneaside and sorbitol although in different amounts, and both compounds varied strongly over the course of the seasons. The seasonal variation in the organic osmolyte concentrations is similar to the results of Karsten and West (2000), who reported three heterosides in the intertidal red alga Bangia atropurpurea following a distinct seasonal pattern with high values in summer and low values in winter. Summer is characterized by more sunshine hours and higher temperatures, which favor photosynthesis and hence carbon fixation for the biosynthesis of organic osmolytes which often represent the primary photosynthetic product in red algae (Karsten 2012). The ability of B. arbuscula to tolerate long periods of desiccation without any major effects on their photosynthetic performance was already shown by Brown (1987). Many Bostrychia species grow in highly exposed habitats, such as the upper intertidal or the epiphytes on mangrove roots, where they experience strong environmental changes and gradients (Karsten 2012). The capability to synthesize and accumulate polyols in high concentrations is considered a biochemical trait which guarantees broad tolerance against salinity and desiccation (Eggert and Karsten 2010). Compared with the digeneaside amounts, the content of sorbitol varied strongly across the different months, which indicates that sorbitol plays the main role in acclimation to salinity stress, which is supported also by other studies (Karsten et al. 1995, 1996; Eggert et al. 2007). Karsten et al. (2005) already showed that digeneaside plays in general a minor role in osmotic acclimation, and hence, its main function is still unknown.
Champia novae-zelandiae synthesized floridoside as the only organic osmolyte, which is typical for many red algae (Karsten et al. 2007). The floridoside concentrations showed strong variation over the course of the seasons. These results might indicate that sunshine and rainfall have a rather minor influence on the floridoside values. However, a comparison of the seasonal changing floridoside amounts with other taxa is difficult due to strong species-specific patterns and a general lack of such annual data (Karsten et al. 1999, 2007; Karsten and West 2000; Eggert et al. 2007; Diehl et al. 2019). C. novae-zelandiae grows in the low intertidal and is for long periods submerged. This suggests that C. novae-zelandiae is less exposed to extreme stress conditions as B. arbuscula in the high intertidal. This is supported by its maximum total organic osmolyte concentrations, which are ca. 30–40% of those in B. arbuscula. Organic osmolyte content is known to correlate with the tidal height of algae (Chen et al. 2014).
The introduced species Schizymenia spp. synthesized floridoside as the main organic osmolyte and the floridoside concentrations varied across the different months with some trend of seasonality. For Schizymenia spp., a complete seasonal sampling set was missing, resulting in a gap for the summer months. In contrast to the two native species (B. arbuscula and C. novae-zelandiae), Schizymenia spp. was collected in an intertidal rock pool, which is less affected by the tides, but could experience salinity changes as well as changes in temperature. In addition, the harbor is much more sheltered than Moa Point, which is located on the south coast of Wellington and therefore more exposed to waves (Morelissen 2012). Some studies indicate that the harbor and Moa Point differ greatly in their intertidal community composition and the human-influenced harbor showed different salinities, dissolved matter and nutrients (Gardner 2000; Helson and Gardner 2004; Fry et al. 2011). All these factors could have an influence on the species-specific metabolic capability to synthesize MAAs and organic osmolytes. Overall, Schizymenia spp. contained lower maximum floridoside concentrations than C. novae-zelandiae. This could be a result of the lower average salinity in harbor compared with Moa Point.
In addition to floridoside, an unknown compound was detected with the HPLC in the monthly samples of Schizymenia spp. This unknown compound was measured in the majority of monthly samples. The percentage proportions of this unknown compound between the different months were almost equal between 30 and 40%. This result could be interpreted as a biochemical advantage if the unknown compound has any protective role and as a result could explain the successful introduction of Schizymenia spp. to New Zealand. Nevertheless, neither the chemical structure nor the function of this unknown compound is clear. The peak of the unknown compound did not match any other known carbohydrate (unpublished data).
Conclusion
This study documented stress metabolite patterns in two native species Bostrychia arbuscula and Champia novae-zelandiae in comparison with the introduced red alga Schizymenia spp. in New Zealand. The hypothesized high MAA concentrations can be confirmed for B. arbuscula, but there were lower levels for C. novae-zelandiae. For the introduced Schizymenia spp., high MAA values can also be found, indicating enhanced UVR tolerance against the solar conditions in New Zealand. Bostrychia arbuscula consistently exhibited two- to threefold higher organic osmolyte concentrations over the course of the year compared with the other species, which matches its high intertidal location. C. novae-zelandiae and Schizymenia spp. contained mainly floridoside, but in much lower amounts, and hence their preferential occurrence in less stressful habitats. Further ecophysiological studies are needed to characterize the tolerance widths of invasive macroalgae in New Zealand as a prognosis tool for further spreading. Additionally, large-scale molecular surveys in combination with ecophysiological studies are needed for red algae in New Zealand to deal with the problems of morphologically indistinguishable species, as in this study, two Schizymenia species (S. apoda and S. dubyi) were found. Based on the chemical analyses and the circumstance that both species grow next to each other in the same habitat, we made the assumption that both species could be physiologically similar, but further analyses are necessary.
References
Adams NM (1994) Seaweeds of New Zealand: an illustrated guide. Canterbury University Press, Christchurch, N.Z
Alexander JM, Edwards PJ (2010) Limits to the niche and range margins of alien species. Oikos 119:1377–1386
Ben-Amotz A, Avron M (1983) Accumulation of metabolites by halotolerant algae and its industrial potential. Annu Rev Microbiol 37:95–119
Bischof K, Rautenberger R (2012) Seaweed responses to environmental stress: reactive oxygen and antioxidative strategies. In: Wiencke C, Bischof K (eds) Seaweed Biology: Novel Insights into Ecophysiology. Ecology and Utilization. Springer, Berlin, pp 109–132
Bischof K, Gómez I, Molis M, Hanelt D, Karsten U, Lüder U, Roleda MY, Zacher K, Wiencke C (2006) Ultraviolet radiation shapes seaweed communities. Rev Env Sci Biotechnol 5:141–166
Bollen M, Pilditch CA, Battershill CN, Bischof K (2016) Salinity and temperature tolerance of the invasive alga Undaria pinnatifida and native New Zealand kelps: implications for competition. Mar Biol 163(9):194
Brown MT (1987) Effects of desiccation on photosynthesis of intertidal algae from a southern New Zealand shore. Bot Mar 30:121–128
Carreto JI, Carignan MO (2011) Mycosporine-like amino acids: relevant secondary metabolites. Chemical and ecological aspects. Mar Drugs 9:387–446
Cheloni G, Slaveykova V (2018) Photo-oxidative stress in green algae and cyanobacteria. Reactive Oxygen Species 5:126–133
Chen J, Song D, Luo Q, Mou T, Yang R, Chen H, He S, Yan X (2014) Determination of floridoside and isofloridoside in red algae by high-performance liquid chromatography-tandem mass spectrometry. Anal Lett 47:2307–2316
CliFlo (National Climate Database) (2017) CliFlo database. https://cliflo.niwa.co.nz/. Accessed 20 January 2020
D’Archino R, Zuccarello GC (2014) First record of Schizymenia apoda (Schizymeniaceae, Rhodophyta) in New Zealand. N Z J Mar Freshw Res 48:155–162
D’Archino R, Zuccarello GC (2020) Schizymenia dubyi (Chauvin ex Duby) J. Agardh collected in Whairepo Lagoon, Wellington Harbour. New Zealand Marine Exotic Species Note 113 March, 2020, pp. 5
D’Archino R, Nelson WA, Zuccarello GC (2007) Invasive marine red alga introduced to New Zealand waters: first record of Grateloupia turuturu (Halymeniaceae, Rhodophyta). N Z J Mar Freshw Res 41:35–42
D’Archino R, Nelson W, Yang MY, Kim MS (2015) New record of Hypnea flexicaulis in New Zealand and description of Calliblepharis psammophilus sp. nov. Bot Mar 58:485–497
Davison IR, Pearson GA (1996) Stress tolerance in intertidal seaweeds. J Phycol 32:197–211
Diehl N, Michalik D, Zuccarello GC, Karsten U (2019) Stress metabolite pattern in the eulittoral red alga Pyropia plicata (Bangiales) in New Zealand – mycosporine-like amino acids and heterosides. J Exp Mar Biol Ecol 510:23–30
Dunlap WC, Yamamoto Y (1995) Small-molecule antioxidants in marine organisms: antioxidant activity of mycosporine-glycine. Comp Biochem PhysiolB 112:105–114
Eggert A, Karsten U (2010) Low molecular weight carbohydrates in red algae – an ecophysiological and biochemical perspective. In: Seckbach J, Chapman DJ (eds) Red Algae in the Genomic Age. Cellular Origin, Life in Extreme Habitats and Astrobiology, vol 13. Springer, Dordrecht, pp 445–456
Eggert A, Nitschke U, West JA, Michalik D, Karsten U (2007) Acclimation of the intertidal red alga Bangiopsis subsimplex (Stylonematophyceae) to salinity changes. J Exp Mar Biol Ecol 343:176–186
Fry B, Rogers K, Barry B, Barr N, Dudley B (2011) Eutrophication indicators in the Hutt River Estuary, New Zealand. N Z J Mar Freshw Res 45:665–677
Gabriel D, Schils T, Parente MI, Draisma SGA, Neto AI, Fredericq S (2011) Taxonomic studies in the Schizymeniaceae (Nemastomatales, Rhodophyta): on the identity of Schizymenia sp. in the Azores and the generic placement of Nemastoma confusum. Phycologia 50:109–121
Garbary DJ, D’Archino R, Flack B, Hepburn CD, Nelson WA, Pritchard D, Sutherland JE (2020) First record of Bonnemaisonia hamifera (Bonnemaisoniales, Rhodophyta) in the South Pacific, from the South Island of New Zealand. N Z J Mar Freshw Res 54:167–176
Gardner JPA (2000) Where are the mussels on Cook Strait (New Zealand) shores? Low seston quality as a possible factor limiting multi-species distributions. Mar Ecol Prog Ser 194:123–132
Greater Wellington Region Council (GWRC ) (2017) Water temperature and rainfall data. http://graphs.gw.govt.nz/?siteName=Wellington. Accessed 20 January 2020
Guiry MD, Guiry GM (2020) AlgaeBase: World-wide electronic publication, National University of Ireland, Galway. https://www.algaebase.org. Accessed 20 January 2020
Gunnarsson K, Russell S, Brodie J (2020) Schizymenia jonssonii sp. nov. (Nemastomatales, Rhodophyta): a relict or an introduction into the North Atlantic after the last glacial maximum? J Phycol 56:324–333
Hanelt D, Figueroa FL (2012) Physiological and photomorphogenic effects of light on marine macrophytes. In: Wiencke C, Bischof K (eds) Seaweed biology: Novel insights into ecophysiology, ecology and utilization. Springer, Berlin, pp 3–23
Hanelt D, Hawes I, Rae R (2006) Reduction of UV-B radiation causes an enhancement of photoinhibition in high light stressed aquatic plants from New Zealand lakes. J Photochem Photobio B 84:89–102
Helson JG, Gardner JPA (2004) Contrasting patterns of mussel abundance at neighbouring sites: does recruitment limitation explain the absence of mussels on Cook Strait (New Zealand) shores? J Exp Mar Biol Ecol 312:285–298
Hoffman JR, Hansen LJ, Klinger T (2003) Interactions between UV radiation and temperature limit inferences from single-factor experiments. J Phycol 39:268–272
Holzinger A, Lütz C, Karsten U, Wiencke C (2004) The effect of ultraviolet radiation on ultrastructure and photosynthesis in the red macroalgae Palmaria palmata and Odonthalia dentata from Arctic waters. Plant Biol 6:568–577
Hoyer K, Karsten U, Sawall T, Wiencke C (2001) Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Mar Ecol Prog Ser 211:117–129
Hoyer K, Karsten U, Wiencke C (2002) Induction of sunscreen compounds in Antarctic macroalgae by different radiation conditions. Mar Biol 141:619–627
Hurd CL, Nelson WA, Falshaw R, Neill KF (2004) History, current status and future of marine macroalgal research in New Zealand: taxonomy, ecology, physiology and human uses. Phycol Res 52:80–106
Karsten U (2008) Defense strategies of algae and cyanobacteria against solar ultraviolet radiation. In: Amsler CD (ed) Algal chemical ecology. Springer, Berlin, pp 273–296
Karsten U (2012) Seaweed acclimation to salinity and desiccation stress. In: Wiencke C, Bischof K (eds) Seaweed biology: Novel insights into ecophysiology, ecology and utilization. Springer, Berlin, pp 87–107
Karsten U, West JA (2000) Living in the intertidal zone — seasonal effects on heterosides and sun-screen compounds in the red alga Bangia atropurpurea (Bangiales). J Exp Mar Biol Ecol 254:221–234
Karsten U, Bock C, West JA (1995) Low molecular weight carbohydrate patterns in geographically different isolates of the eulittoral red alga Bostrychia tenuissima from Australia. Bot Acta 108:321–326
Karsten U, Koch S, West JA, Kirst GO (1996) Physiological responses of the eulittoral macroalga Stictosiphonia hookeri (Rhodomelaceae, Rhodophyta) from Argentina and Chile: salinity, light and temperature acclimation. Eur J Phycol 31:361–368
Karsten U, Barrow KD, Nixdorf O, West JA, King RJ (1997) Characterization of mannitol metabolism in the mangrove red alga Caloglossa leprieurii (Montagne) J. Agardh. Planta 201:173–178
Karsten U, Sawall T, Hanelt D, Bischof K, Figueroa FL, Flores-Moya A, Wiencke C (1998a) An inventory of UV-absorbing mycosporine-like amino acids in macroalgae from polar to warm-temperate regions. Bot Mar 41:443–454
Karsten U, Franklin LA, Lüning K, Wiencke C (1998b) Natural ultraviolet radiation and photosynthetically active radiation induce formation of mycosporine-like amino acids in the marine macroalga Chondrus crispus (Rhodophyta). Planta 205:257–262
Karsten U, West JA, Zuccarello GC, Nixdorf O, Barrow KD, King RJ (1999) Low molecular weight carbohydrate patterns in the Bangiophyceae (Rhodophyta). J Phycol 35:967–976
Karsten U, Michalik D, Michalik M, West JA (2005) A new unusual low molecular weight carbohydrate in the red algal genus Hypoglossum (Delesseriaceae, Ceramiales) and its possible function as an osmolyte. Planta 222:319–326
Karsten U, Görs S, Eggert A, West JA (2007) Trehalose, digeneaside, and floridoside in the Florideophyceae (Rhodophyta) – a reevaluation of its chemotaxonomic value. Phycologia 46:143–150
Karsten U, Escoubeyrou K, Charles F (2009) The effect of redissolution solvents and HPLC columns on the analysis of mycosporine-like amino acids (MAAs) in the macroalgal species Prasiola crispa and Porphyra umbilicalis. Helgol Mar Res 63:231–238
Kirst GO (1990) Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Physiol 41:21–53
Korbee N, Huovinen P, Figueroa FL, Aguilera J, Karsten U (2005) Availability of ammonium influences photosynthesis and the accumulation of MAAs in two Porphyra species (Bangiales, Rhodophyta). Mar Biol 146:645–654
Lamare MD, Lesser MP, Barker MF, Barry TM, Schimanski KB (2004) Variation in sunscreen compounds (mycosporine-like amino acids) for marine species along a gradient of ultraviolet radiation transmission within doubtful sound, New Zealand. N Z J Mar Freshw Res 38:775–793
Lüning K (1990) Seaweeds: their environment, biogeography, and ecophysiology. Wiley, New York
McKenzie R, Connor B, Bodeker G (1999) Increased summertime UV radiation in New Zealand in response to ozone loss. Science 285:1709–1711
McKenzie R, Smale D, Kotkamp M (2004) Relationship between UVB and erythemally weighted radiation. Photochem Photobiol Sci 3:252–256
Morelissen B (2012) Ecological effects of Undaria pinnatifida (Harvey) Suringar and nutrient-enrichment on intertidal assemblages in the Wellington region of New Zealand. PhD dissertation, Victoria University of Wellington
Muangmai N, Preuss M, Zuccarello GC (2015) Comparative physiological studies on the growth of cryptic species of Bostrychia intricata (Rhodomelaceae, Rhodophyta) in various salinity and temperature conditions. Phycol Res 63:300–306
Nakamura H, Kobayashi J’i, Hirata Y (1982) Separation of mycosporine-like amino acids in marine organisms using reversed-phase high-performance liquid chromatography. J Chromatogr A 250:113–118
National Institute for Water and Atmosphere (NIWA) (2016) UV and ozone. https://www.niwa.co.nz/our-services/online-services/uv-ozone. Accessed 20 January 2020
Nelson W (2013) New Zealand seaweeds: an illustrated guide. Te Papa Press, Wellington, New Zealand
Nelson WA, Kim SY, D’Archino R, Boo SM (2013) The first record of Grateloupia subpectinata from the New Zealand region and comparison with G. prolifera, a species endemic to the Chatham Islands. Bot Mar 56:507–513
Orfanoudaki M, Hartmann A, Karsten U, Ganzera M (2018) Chemical profiling of mycosporine-like amino acids in twenty-three red algal species. J Phycol
Orfanoudaki M, Hartmann A, Miladinovic H, Nguyen Ngoc H, Karsten U, Ganzera M (2019) Bostrychines A-F, six novel mycosporine-like amino-acids and a novel betaine from the red alga Bostrychia scorpioides. Mar Drugs 17:356
Physiology University of Guelph (2008) Crop physiology: introduction: solar radiation and irradiance. https://www.uoguelph.ca/plant/courses/pbio-3110/lectures/lec05_08_000.pdf. Accessed 6 January 2017
Ramirez ME, Nuñez JD, Ocampo EH, Matula CV, Suzuki M, Hashimoto T, Cledón M (2012) Schizymenia dubyi (Rhodophyta, Schizymeniaceae), a new introduced species in Argentina. N Z J Bot 50:51–58
Russell G (1987) Salinity and seaweed vegetation. In: Crawford RMM (ed) The physiological ecology of amphibious and intertidal plants. Blackwell, Oxford, pp 35–52
Saunders GW, Birch TC, Dixon KR (2015) A DNA barcode survey of Schizymenia (Nemastomatales, Rhodophyta) in Australia and British Columbia reveals overlooked diversity including S. tenuis sp. nov. and Predaea borealis sp. nov. Botany 93:859–871
Schaffelke B, Smith JE, Hewitt CL (2006) Introduced macroalgae – a growing concern. J Appl Phycol 18:529–541
Schweikert K, Sutherland JES, Hurd CL, Burritt DJ (2011) UV-B radiation induces changes in polyamine metabolism in the red seaweed Porphyra cinnamomea. Plant Growth Regul 65:389–399
Shetty P, Gitau MM, Maróti G (2019) Salinity stress responses and adaptation mechanisms in eukaryotic green microalgae. Cells 8:1657
Sudhir P, Murthy SDS (2004) Effects of salt stress on basic processes of photosynthesis. Photosynthetica 42:481–486
Underwood AJ (1997) Analysis of variance in experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge pp 140–197
Vass I, Szilárd A, Sicora C (2005) Adverse effects of UV-B light on the structure and function of the photosynthetic apparatus. In: Pessarakli M (ed) Handbook of Photosynthesis. Taylor and Francis, London, pp 43–63
Acknowledgments
Open Access funding enabled and organized by Projekt DEAL. Vanessa Gambichler thanks the DAAD for a PROMOS Scholarship supporting her research stay in New Zealand. We thank Maren Preuss for her help collecting samples for this study, and all her intellectual input, as well as John van der Sman and Neville Higgison for their technical support in the lab. We also thank Julianne Müller for her support with the HPLC and Rhena Schuhmann for helping with HPLC-Quality Management.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 444 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gambichler, V., Zuccarello, G.C. & Karsten, U. Seasonal changes in stress metabolites of native and introduced red algae in New Zealand. J Appl Phycol 33, 1157–1170 (2021). https://doi.org/10.1007/s10811-020-02365-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02365-0