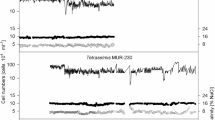
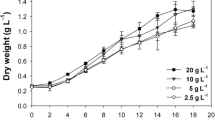
Abstract
Chlorella sorokiniana (DOE 1412) emerged as one of the most promising microalgae strains from the NAABB consortium project and was found to have a remarkable doubling time under optimal conditions of 2.57 h−1. However, its maximum achievable annual biomass productivity in outdoor ponds in the contiguous USA has not yet been demonstrated. In order to address this knowledge gap, this alga was cultured in indoor LED-lighted and temperature-controlled raceways in nutrient replete freshwater (BG-11) medium at pH 7 under conditions simulating the daily sunlight intensity and water temperature fluctuations during three seasons in Southern Florida, an optimal outdoor pond culture location for this organism identified by prior biomass growth modeling. Prior strain characterization indicated that the average maximum specific growth rate (μ max) at 36 °C declined continuously with pH, with μ max corresponding to 5.92, 5.83, 4.89, and 4.21 day−1 at pH 6, 7, 8, and 9, respectively. In addition, the maximum specific growth rate declined nearly linearly with increasing salinity until no growth was observed above 35 g L−1 NaCl. In the climate-simulated culturing studies, the volumetric ash-free dry weight-based biomass productivities during the linear growth phase were 57, 69, and 97 mg L−1 day−1 for 30-year averaged light and temperature simulations for January (winter), March (spring), and July (summer), respectively, which correspond to average areal productivities of 11.6, 14.1, and 19.9 g m−2 day−1. The photosynthetic efficiencies (PAR) in these three climate-simulated pond culturing experiments ranged from 4.1 to 5.1%. The annual biomass productivity was estimated as ca. 15 g m−2 day−1, nearly double the US Department of Energy (DOE) 2015 State of Technology annual cultivation productivity of 8.5 g m−2 day−1, but still well below the projected DOE 2022 target of ca. 25 g m−2 day−1 required for economic microalgal biofuel production, indicating the need for additional research.






Similar content being viewed by others
References
Batterton JC, Baalen CV (1971) Growth response of blue-green algae to sodium chloride concentration. Arch Microbiol 76:151–165
Bechet Q, Munoz R, Shilton A, Guieysse B (2013) Outdoor cultivation of temperature-tolerant Chlorella sorokiniana in a column photobioreactor under low power-input. Biotechnol Bioeng 110:118–126
Convalves AMM, de Figueiredo DR, Pereira MJ (2005) The effects of different salinity concentrations on growth of three freshwater green algae. Fresenius Environ Bull 15:1382–1386
Cuaresma M, Janssen M, Vilchez C, Wijffels RH (2009) Productivity of Chlorella sorokiniana in a short light-path (SLP) panel photobioreactor under high irradiance. Biotechnol Bioeng 104:352–359
Cuaresma-Franco M, Buffing MF, Janssen M, Vílchez Lobato C, Wijffels RH (2012) Performance of Chlorella sorokiniana under simulated extreme winter conditions. J Appl Phycol 24:693–699
Doucha J, Livansky K (2006) Productivity, CO2/O2 exchange and hydraulics in outdoor open high density microalgal (Chlorella sp.) photobioreactors operated in a middle and southern European climate. J Appl Phycol 18:811–826
Doucha J, Livansky K (2009) Outdoor open thin-layer microalgal photobioreactor: potential productivity. J Appl Phycol 21:111–117
Edmundson SJ, Huesemann MH (2015) The dark side of algae cultivation: characterizing night biomass loss in three photosynthetic algae, Chlorella sorokiniana, Nannochloropsis salina, and Picochlorum sp. Algal Res 12:470–476
Fabregas J, Abalde J, Herrero C, Cabezas BV, Veiga M (1984) Growth of the marine microalga Tetraselmis suecica in batch cultures with different salinities and nutrient concentrations. Aquaculture 42:207–215
Goldman JC, Azov Y, Riley CB, Dennett MR (1982) The effect of pH in intensive microalgal cultures. I. Biomass regulation. J Exp Mar Biol Ecol 57:1–13
Hase R, Oiakawa H, Sasa C, Morita M, Watanabe Y (2000) Photosynthetic production of microalgal biomass in a raceway system under greenhouse conditions in Sendai City. J Biosci Bioeng 89:157–163
Huesemann MH, Van Wagenen J, Miller T et al (2013) A screening model to predict microalgae biomass growth in photobioreactors and ponds. Biotechnol Bioeng 111:1583–1594
Huesemann MH, Crowe B, Waller P, Chavis A, Hobbs S, Edmundson S, Wigmosta M (2016) A validated model to predict microalgae growth in outdoor pond cultures subjected to fluctuating light intensities and water temperatures. Algal Res 13:195–206
Huesemann M, Dale T, Chavis A, Crowe B, Twary S, Barry A, Valentine D, Yoshida R, Wigmosta M, Cullinan V (2017) Simulation of outdoor pond cultures using indoor LED-lighted and temperature-controlled raceway ponds and Phenometrics™ photobioreactors. Algal Res 21:178–190
Jensen S, Knutsen G (1993) Influence of light and temperature on photoinhibition of photosynthesis in Spirulina platensis. J Appl Phycol 5:495–504
Kaewkannetra P, Enmak P, Chiu TY (2012) The effect of CO2 and salinity on the cultivation of Scenedesmus obliquus for biodiesel production. Biotechnol Bioprocess Eng 17:591–597
Kotrbacek V, Doubek J, Doucha J (2015) The chlorococcalean alga Chlorella in animal nutrition: a review. J Appl Phycol 27:2173–2180
Lammers PJ, Huesemann M, Boeing W, Anderson DB, Arnold RG, Bai X, Bhole M, Brhanavan Y, Brown L, Brown J, Brown JK, Chisholm S, Meghan Downes C, Fulbright S, Ge Y, Holladay JE, Ketheesan B, Khopkar A, Koushik A, Laur P, Marrone BL, Mott JB, Nirmalakhandan N, Ogden KL, Parsons RL, Polle J, Ryan RD, Samocha T, Sayre RT, Seger M, Selvaratnam T, Sui R, Thomasson A, Unc A, Van Voorhies W, Waller P, Yao Y, Olivares JA (2017) Review of the cultivation program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Res 22:166–186
Lucker BF, Hall CC, Zegarac R, Kramer DM (2014) The environmental photobioreactor (ePBR): an algal culturing platform for simulating dynamic natural environments. Algal Res 6:242–249
Moheimani NR (2013) Inorganic carbon and pH effect on growth and lipid productivity of Tetraselmis sueica and Chlorella sp. (Chlorophyta) grown outdoors in bag photobioreactors. Jf Appl Phycol 25:387–398
Morita M, Wanatabe Y, Saiki H (2000) High photosynthetic productivity of green microalga Chlorella sorokiniana. Appl Biochem Biotechnol 87:203–218
Neofotis P, Huang A, Sury K, Chang W, Joseph F, Gabr A, Twary S, Qiu W, Holguine O, Polle JEW (2016) Characterization and classification of highly productive microalgae strains discovered for biofuel and bioproduct generation. Algal Res 15:164–178
Perkins W A, Richmond MC (2004) MASS2, Modular Aquatic Simulation System in Two Dimensions: theory and numerical methods. Report PNNL-14820-1, Pacific Northwest National Laboratory, Richland, Washington
Rachlin JW, Grosso A (1991) The effects of pH on the growth of Chlorella vulgaris and its interactions with cadmium toxicity. Arch Environ Contam Toxicol 20:505–508
Renaud S, Parry D (1994) Microalgae for use in tropical aquaculture II: effect of salinity on growth, gross chemical composition and fatty acid composition of three species of marine microalgae. J Appl Phycol 6:347–356
Sogaard DH, Hansen PJ, Rysgaard S, Glud RN (2011) Growth limitation of three Arctic Sea ice algae species: effects of salinity, pH, and inorganic carbon availability. Polar Biol 34:1157–1165
Sorokin C (1959) Tabular comparative data for the low- and high-temperature strains of Chlorella. Nature 185:613–614
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Unkefer CJ, Sayre RT, Magnuson JK, Anderson DB, Baxter I, Blaby IK, Brown JK, Carleton M, Cattolico RA, Dale T, Devarenne TP, Downes CM, Dutcher SK, Fox DT, Goodenough U, Jaworski J, Holladay JE, Kramer DM, Koppisch AT, Lipton MS, Marrone BL, McCormick M, Molnár I, Mott JB, Ogden KL, Panisko EA, Pellegrini M, Polle J, Richardson JW, Sabarsky M, Starkenburg SR, Stormo GD, Teshima M, Twary SN, Unkefer PJ, Yuan JS, Olivares JA (2017) Review of the algal biology program within the National Alliance for Advanced Biofuels and Bioproducts. Algal Res 22:187–215
US Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy (2016) Bioenergy Technology Office—Multi-year program plan, page A-2. https://www.energy.gov/eere/bioenergy/downloads/bioenergy-technologies-office-multi-year-program-plan-march-2016
US Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, Biomass Program (2010) National algal biofuels technology roadmap. https://doi.org/10.2172/1218560. https://energy.gov/eere/bioenergy/downloads/national-algal-biofuels-technology-roadmap
Van Auken OW, McNulty IB (1973) The effect of environmental factors on the growth of a halophilic species of algae. Biol Bull 145:210–222
Van Wagenen J, Miller TW, Hobbs S, Hook P, Crowe B, Huesemann MH (2012) Effects of light intensity and temperature on fatty acid composition in Nannochloropsis salina. Energies 5:731–740
Vonshak A, Torzillo G, Masojidek J, Boussiba S (2001) Sub-optimal morning temperature induces photoinhibition in dense outdoor cultures of the alga Monodus subterraneous (Eustigmatophyta). Plant Cell Environ 24:1113–1118
Wigmosta MS, Coleman AM, Skaggs RJ, Huesemann MH, Lane LJ (2011) National microalgae biofuels production potential and resource demand. Water Resour Res 47(3). https://doi.org/10.1029/2010WR009966
Williams PJB, Laurens LML (2010) Microalgae as biodiesel & biomass feedstocks: review & analysis of the biochemistry, energetics, & economics. Energy Environ Sci 3:554–590
Yandu L, Chi X, Li Z, Yang Q, Li F, Liu S, Gan Q, Qin S (2010) Isolation and characterization of a stress-dependent plastidial D12 fatty acid desaturase from the Antarctic microalga Chlorella vulgaris NJ-7. Lipids 45:179–187
Acknowledgements
This research was funded by the Bioenergy Technologies Office, US Department of Energy (Agreements DE-EE0006316 and DE-EE0006317), via subcontracts from New Mexico State University and California Polytechnic State University, San Luis Obispo, respectively. Additional support by the Department of Energy Science Undergraduate Laboratory Internship Program was provided to David Rye and Samuel Hobbs.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 3.70 mb)
Rights and permissions
About this article
Cite this article
Huesemann, M., Chavis, A., Edmundson, S. et al. Climate-simulated raceway pond culturing: quantifying the maximum achievable annual biomass productivity of Chlorella sorokiniana in the contiguous USA. J Appl Phycol 30, 287–298 (2018). https://doi.org/10.1007/s10811-017-1256-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1256-6