Abstract
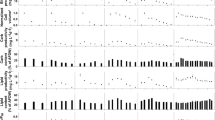
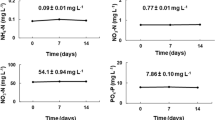
Microalgae are ideal candidates for bioremediation and biotechnological applications. However, salinity and nutrient resource availability vary seasonally and between cultivation sites, potentially impacting on biomass productivity. The aim of this study was to screen pollutant-tolerant freshwater microalgae (Desmodesmus armatus, Mesotaenium sp., Scenedesmus quadricauda and Tetraedron sp.), isolated from Tarong power station ash-dam water, for their tolerance to cultivation at a range of salinities. To determine if biochemical composition could be manipulated, the effects of 4-day nutrient limitation were also determined. Microalgae were cultured at 2, 8, 11 and 18 ppt salinity, and nutrient uptake was monitored daily. Growth, total lipid, fatty acid (FA), and amino acid contents were quantified in biomass harvested while nutrient-replete and, after 4 days, nutrient-deplete. D. armatus showed the highest salinity tolerance actively growing in up to 18 ppt while Mesotaenium sp. was the least halotolerant with decreasing growth rates from 11 ppt. However, Mesotaenium sp. at 2 and 8 ppt had the highest biomass productivity and nutrient requirements of the four species, making it ideal for nutrient remediation of eutrophic freshwater effluents. Salinity and nutrient status had minimal influence on total lipid and FA contents in D. armatus and Mesotaenium sp., while nutrient depletion induced an increase of total lipid and FAs in S. quadricauda and Tetraedron sp., which was further increased with increasing salinity. As none of the growth conditions affected amino acid profiles of the species, these findings provide a basis for species selection based on site-specific salinity conditions and nutrient resource availability.




Similar content being viewed by others
References
Ahlgren G, Hyenstrand P (2003) Nitrogen limitation effects of different nitrogen sources on nutritional quality of two freshwater organisms, Scenedesmus quadricauda (Chlorophyceae) and Synechococcus sp. (Cyanophyceae). J Phycol 39:906–917
Aitchison PA, Butt VS (1973) Relation between synthesis of inorganic polyphosphate and phosphate uptake by Chlorella vulgaris. J Exp Bot 24:497–510
Andersen RA, Berges JA, Harrison PJ, Watanabe MM (2005) Recipes for freshwater and saltwater media. In: Andersen RA (ed) Algal culturing techniques. Elsevier, London, pp 429–538
Aravantinou AF, Theodorakopoulos MA, Manariotis ID (2013) Selection of microalgae for wastewater treatment and potential lipids production. Bioresour Technol 147:130–134
Becker EW (1994) Microalgae: biotechnology and microbiology. Cambridge University Press, New York
Ben-Amotz A, Avron M (1983) Accumulation of metabolites by halotolerant algae and its industrial potential. Annu Rev Microbiol 37:95–119
Ben-Amotz A, Tornabene TG, Thomas WH (1985) Chemical profile of selected species of microalgae with emphasis on lipids. J Phycol 21:72–81
Boland MJ et al (2013) The future supply of animal-derived protein for human consumption. Trends Food Sci Technol 29:62–73
BOM (2006) Average pan evaporation December. Bureau of Meteorology, http://www.bom.gov.au/jsp/ncc/climate_averages/evaporation/index.jsp?period=dec#maps
Borowitzka M, Moheimani N (2013) Sustainable biofuels from algae Mitigat Adaptat. Strat Global Change 18:13–25
Brown AD (1976) Microbial water stress. Bact Rev 40:803–846
Brown LM (1982) Photosynthetic and growth responses to salinity in a marine isolate of Nannochloris bacillaris (Chlorophyceae). J Phycol 18:483–488
Brown MR (1991) The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J Exp Mar Biol Ecol 145:79–99
Brown LM, Hellebust JA (1978) Sorbitol and proline as intracellular osmotic solutes in the green alga Stichococcus bacillaris. Can J Bot 56:676–679
Brown MR, Jeffrey SW (1992) Biochemical composition of microalgae from the green algal classes Chlorophyceae and Prasinophyceae. 1. Amino acids, sugars and pigments. J Exp Mar Biol Ecol 161:91–113
Brune DE, Lundquist TJ, Beneman JR (2009) Microalgal biomass for greenhouse gas reductions: potential for replacement of fossil fuels and animal feeds. J Env Eng 135:1136–1144
Carvalho AP, Meireles LA, Malcata FX (1998) Rapid spectrophotometric determination of nitrates and nitrites in marine aqueous culture media. Analusis 26:347–351
Chan A, Salsali H, McBean E (2014) Nutrient removal (nitrogen and phosphorous) in secondary effluent from a wastewater treatment plant by microalgae. Can J Civil Eng 41:118–124
Cheng Y-S, Zheng Y, Labavitch JM, Vander Gheynst JS (2011) The impact of cell wall carbohydrate composition on the chitosan flocculation of Chlorella Process. Biochem 46:1927–1933
Clarke KR (1993) Nonparametric multivariate analyses of changes in community structure Australian. J Ecol 18:117–143
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151
Cordell D, Drangert J-O, White S (2009) The story of phosphorus: global food security and food for thought Global. Env Change 19:292–305
Dawson CJ, Hilton J (2011) Fertiliser availability in a resource-limited world: production and recycling of nitrogen and phosphorus. Food Policy 36(Suppl 1):S14–S22.
de Jesus Raposo MF, de Morais RMSC, de Morais AMMB (2013) Health applications of bioactive compounds from marine microalgae. Life Sci 93:479–486
De Silva S, Turchini G, Francis D (2012) Nutrition. In: Lucas JS, Southgate PC (eds) Aquaculture: farming aquatic animals and plants. Second edn. Blackwell, Oxford, pp 164–187
Dean AP, Sigee DC, Estrada B, Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Biores Technol 101:4499–4507
Dickinson KE, Whitney CG, McGinn PJ (2013) Nutrient remediation rates in municipal wastewater and their effect on biochemical composition of the microalga Scenedesmus sp. AMDD Algal Res 2:127–134
D’Mello JPF (1993) Amino acid supplementation of cereal-based diets for non-ruminants animal feed. Sci Technol 45:1–18
Dortch Q, Clayton JR Jr, Thoresen SS, Ahmed SI (1984) Species differences in accumulation of nitrogen pools in phytoplankton. Mar Biol 81:237–250
Draaisma RB, Wijffels RH, Slegers PM, Brentner LB, Roy A, Barbosa MJ (2013) Food commodities from microalgae. Curr Opin Biotech 24:169–177
Dunstan GA, Volkman JK, Jeffrey SW, Barrett SM (1992) Biochemical composition of microalgae from the green algal classes Chlorophyceae and Prasinophyceae. 2. Lipid classes and fatty acids. J Exp Mar Biol Ecol 161:115–134
Eixler S, Karsten U, Selig U (2006) Phosphorus storage in Chlorella vulgaris (Trebouxiophyceae, Chlorophyta) cells and its dependence on phosphate supply. Phycologia 45:53–60
Erdmann N, Hagemann M (2001) Salt acclimation of algae and cyanobacteria: a comparison. In: Rai LC, Gaur JP (eds) Algal Adaptation to environmental stresses. Springer, Berlin, pp 323–361
Flynn KJ (1990) Composition of intracellular and extracellular pools of amino acids, and amino acid utilization of microalgae of different sizes. J Exp Mar Biol Ecol 139:151–166
Gao Y, Yang M, Wang C (2013) Nutrient deprivation enhances lipid content in marine microalgae. Bioresour Technol 147:484–491
Garcia-Moscoso JL, Obeid W, Kumar S, Hatcher PG (2013) Flash hydrolysis of microalgae (Scenedesmus sp.) for protein extraction and production of biofuels intermediates. J Supercrit Fluids 82:183–190
Gosch BJ, Magnusson M, Paul NA, de Nys R (2012) Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts global change. Biol Bioenergy 4:919–930
Greenway H, Setter TL (1979) Accumulation of proline and sucrose during the 1st hours after transfer of Chlorella emersonii to high NaCl. Aus J Plant Physiol 6:69–79
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as sources of carotenoids. Mar Drugs 9:625–644
Guil-Guerrero JL (2007) Stearidonic acid (18:4n-3): metabolism, nutritional importance, medical uses and natural sources. Eur J Lipid Sci Technol 109:1226–1236
Guiry MD (2012) How many species of algae are there? J Phycol 48:1057–1063
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Hart B, Bailey P, Edwards R, Hortle K, James K, McMahon A, Meredith C, Swadling K (1991) A review of the salt sensitivity of the Australian freshwater biota. Hydrobiologia 210:105–144
Ho S-H, Chen C-Y, Lee D-J, Chang J-S (2011) Perspectives on microalgal CO2-emission mitigation systems—a review. Biotechnol Adv 29:189–198
Ho S-H, Chen C-Y, Chang J-S (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252
Imamoglu E, Dalay MC, Sukan FV (2009) Influences of different stress media and high light intensities on accumulation of astaxanthin in the green alga Haematococcus pluvialis. New Biotechnol 26:199–204
Kirst GO (1989) Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Physiol 41:21–53
Krienitz L, Takeda H, Hepperle D (1999) Ultrastructure, cell wall composition, and phylogenetic position of Pseudodictyosphaerium jurisii (Chlorococcales, Chlorophyta) including a comparison with other picoplanktonic green algae. Phycologia 38:100–107
Kung L Jr, Rode LM (1996) Amino acid metabolism in ruminants animal feed. Sci Technol 59:167–172
Lang IK, Hodac L, Friedl T, Feussner I (2011) Fatty acid profiles and their distribution patterns in microalgae: a comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol 11:124
Lim DK, Garg S, Timmins M, Zhang ES, Thomas-Hall SR, Schuhmann H, Li Y, Schenk PM (2012) Isolation and evaluation of oil-producing microalgae from subtropical coastal and brackish waters. PLoS One 7(7), e40751
Lligadas G, Ronda JC, Galia M, Cadiz V (2010) Oleic and undecylenic acids as renewable feedstocks in the synthesis of polyols and polyurethanes. Polymers 2:440–453
MacIntyre HL, Cullen JJ (2005) Using cultures to investigate the physiological ecology of microalgae. In: Andersen RA (ed) Algal culturing techniques. Elsevier, London, pp 287–326
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Malerba ME, Connolly SR, Heimann K (2012) Nitrate-nitrite dynamics and phytoplankton growth: formulation and experimental evaluation of a dynamic model Limnol Oceanogr 57
Martı́nez ME, Sánchez S, Jiménez JM, El Yousfi F, Muñoz L (2000) Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour Technol 73:263–272
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review Renew. Sust Energy Rev 14:217–232
Muller D, Forster D, Magert HJ, Grewe C, Griehl C (2005) Astaxanthin accumulation under specific stress conditions in Scenedesmus strains. Phycologia 44:39–39
Nunes AJP, Sá MVC, Browdy CL (2014) Practical supplementation of shrimp and fish feeds with crystalline amino acids. Aquaculture 431:20–27
Olofsson M, Lamela T, Nilsson E, Bergé J-P, del Pino V, Uronen P, Legrand C (2014) Combined effects of nitrogen concentration and seasonal changes on the production of lipids in Nannochloropsis oculata. Mar Drugs 12:1891–1910
Oren A (1999) Bioenergetic aspects of halophilism. Microbiol Molec Biol Rev 63:334–348
Patil HS (1991) The role of Ankistrodesmus falcatus and Scenedesmus quadricauda in sewage purification. Bioresour Technol 37:121–126
Peck AJ, Hatton T (2003) Salinity and the discharge of salts from catchments in Australia. J Hydrol 272:191–202
Powell N, Shilton A, Chisti Y, Pratt S (2009) Towards a luxury uptake process via microalgae - defining the polyphosphate dynamics. Water Res 43:4207–4213
Proksch E, Holleran WM, Menon GK, Elias PM, Feingold KR (1993) Barrier function regulates epidermal lipid and DNA synthesis Brit. J Dermatol 128:473–482
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Rao AR, Dayananda C, Sarada R, Shamala TR, Ravishankar GA (2007) Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol 98:560–564
Rawles SD, Fuller SA, Beck BH, Gaylord TG, Barrows FT, McEntire ME (2013) Lysine optimization of a commercial fishmeal-free diet for hybrid striped bass (Morone chrysops x M. saxatilis). Aquaculture 396–399:89–101
Reitan KI, Rainuzzo JR, Olsen Y (1994) Effect of nutrient limitation on fatty-acid and lipid content of marine microalgae. J Phycol 30:972–979
Renaud SM, Parry DL (1994) Microalgae for use in tropical aquaculture 2. Effects of salinity on growth, gross-chemical composition and fatty acid composition of three species of marine microalgae. J Appl Phycol 6:347–356
Richardson JTE (2011) Eta squared and partial eta squared as measures of effect size in educational research. Educa Res Rev 6:135–147
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotech Bioeng 102:100–112
Salama E-S, Abou-Shanaba RA, Kim JR, Lee S, Kim SH, Oh SE, Kim HC, Roh HS, Jeon BH (2014) The effects of salinity on the growth and biochemical properties of Chlamydomonas mexicana GU732420 cultivated in municipal wastewater. Env Technol 35:1491–1498
Setter TL, Greenway H (1979) Growth and osmoregulation of Chlorella emersonii in NaCl and neutral osmotica. Aust J Plant Physiol 6:47–60
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553
Shi J, Podola B, Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19:417–423
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids Biomed. Pharmacotherapy 56:365–379
Sims GG (1978) Rapid estimation of carbohydrate in formulated fish products - protein by difference. J Sci Food Agric 29:281–284
Stephens E, Ross IL, Mussgnug JH, Wagner LD, Borowitzka MA, Posten C, Kruse O, Hankamer B (2010) Future prospects of microalgal biofuel production systems. Trends Plant Sci 15:554–564
Su CH, Chien LJ, Gomes J, Lin YS, Yu YK, Liou JS, Syu RJ (2011) Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. J Appl Phycol 23:903–908
Sudhir P, Murthy SDS (2004) Effects of salt stress on basic processes of photosynthesis. Photosynthetica 42:481–486
Tiftickjian JD, Rayburn WR (1986) Nutritional requirements for sexual reproduction in Mesotaenium kramstai (Chlorophyta). J Phycol 22:1–8
Van Veldhoven PP, Mannaerts GP (1987) Inorganic and organic phosphate measurements in the nanomolar range. Anal Biochem 161:45–48
Vanlerberghe GC, Brown LM (1987) Proline overproduction in cells of the green alga Nannochloris bacillaris resistant to azetidine 2 carboxylic group. Plant Cell Env 10:251–257
von Alvensleben N, Stookey K, Magnusson M, Heimann K (2013) Salinity tolerance of Picochlorum atomus and the use of salinity for contamination control by the freshwater cyanobacterium Pseudanabaena limnetica. PLoS One 8(5), e63569
Williams PJB, Laurens LML (2010) Microalgae as biodiesel & biomass feedstocks: review & analysis of the biochemistry, energetics & economics energy. Env Sci 3:554–590
Xia L, Rong J, Yang H, He Q, Zhang D, Hu C (2014) NaCl as an effective inducer for lipid accumulation in freshwater microalgae Desmodesmus abundans. Bioresour Technol 161:402–409 d
Zhila NO, Kalacheva GS, Volova TG (2005) Influence of nitrogen deficiency on biochemical composition of the green alga Botryococcus. J Appl Phycol 17:309–315 d
Zhila NO, Kalacheva GS, Volova TG (2011) Effect of salinity on the biochemical composition of the alga Botryococcus braunii Kütz IPPAS H-252. J Appl Phycol 23:47–52
Zhou WG, Li YC, Min M, Hu B, Chen P, Ruan R (2011) Local bioprospecting for high-lipid producing microalgal strains to be grown on concentrated municipal wastewater for biofuel production. Bioresour Technol 102:6909–6919
Acknowledgments
The project was supported by the Advanced Manufacturing Cooperative Research Centre (AMCRC), funded through the Australian Government’s Cooperative Research Centre Scheme, grant number 2.2.2. The funders had no role in study design, data collection and analysis or preparation of the manuscript and have provided permission to publish. This research is part of the MBD Energy Research and Development program for Biological Carbon Capture and Storage. Nicolas von Alvensleben was supported by an AMCRC PhD scholarship.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
S. Table 1
Effect of salinity and culture nutrient status (replete/deplete) on Desmodesmus armatus fatty acid profiles (FA content (mg g-1 DW)) (DOCX 18 kb)
S. Table 2
Effect of salinity and culture nutrient status (replete/deplete) on Mesotaenium sp. fatty acid profiles (FA content [mg g-1 DW]) (DOCX 17 kb)
S. Table 3
Effect of salinity and culture nutrient status (replete/deplete) on Scenedesmusquadricauda fatty acid profiles (FA content [mg g-1 DW]) (DOCX 16 kb)
S. Table 4
Effect of salinity and culture nutrient status (replete/deplete) on Tetraedron sp. fatty acid profiles (FA content [mg g-1 DW]) (DOCX 17 kb)
S. Table 5
Total lipid and total FAME productivities [mg L-1 day-1] ofDesmodesmus armatus, Mesotaeniumsp., Scenedesmus quadricauda and Tetraedron sp. at 2, 8, 11 and 18 ppt salinity. Productivities were derived from biomass productivities during the exponential growth phase. (DOCX 15 kb)
S. Table 6
Individual FAME productivities [mg L-1 day-1] ofDesmodesmus armatus, Mesotaeniumsp., Scenedesmus quadricauda and Tetraedron sp. at 2, 8, 11 and 18 ppt salinity. Productivities were derived from biomass productivities during the exponential growth phase. (DOCX 17 kb)
S. Table 7
Amino acid profiles [mg g-1 DW] of Desmodesmus armatus at 2 and 11 ppt in nutrient-replete and deplete conditions. (DOCX 15 kb)
S. Table 8
Amino acid profiles [mg g-1 DW] of Mesotaeniumsp. at 2 and 11 ppt in nutrient-replete and deplete conditions. (DOCX 15 kb)
S. Table 9
Amino acid profiles [mg g-1 DW] of Scenedesmus quadricauda at 2 and 11 ppt in nutrient-replete and deplete conditions. (DOCX 15 kb)
S. Table 10
Amino acid profiles [mg g-1 DW] of Tetraedronsp. at 2 and 11 ppt in nutrient-replete and deplete conditions. (DOCX 15 kb)
Rights and permissions
About this article
Cite this article
von Alvensleben, N., Magnusson, M. & Heimann, K. Salinity tolerance of four freshwater microalgal species and the effects of salinity and nutrient limitation on biochemical profiles. J Appl Phycol 28, 861–876 (2016). https://doi.org/10.1007/s10811-015-0666-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0666-6