Abstract
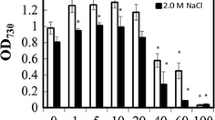
γ-Aminobutyric acid (GABA) is known as an inhibitory neurotransmitter in human, while in plants, GABA is an intermediate for amino acid metabolism and also is accumulated in response to a wide range of environmental stress. In the present study, GABA accumulation in Aphanothece halophytica was increased 2-fold in mid-log phase cells grown under salt stress (2.0 M NaCl). When mid-log phase cells were subjected to changes in NaCl concentrations and pH for 4 h, the highest GABA accumulation was observed in cells adapted in medium that contained 2.0 M NaCl and that was adjusted to pH 4.0, respectively. The increase of GABA accumulation was accompanied by an increased glutamate decarboxylase activity. Addition of glutamate to growth medium stimulated GABA accumulation under acid stress but had no effect under salt stress. However, the highest GABA accumulation was detected in cells exposed to both high salt and acid stresses combined with the 5 mM glutamate supplementation with an approximately 3-fold increase as compared to the control. The unicellular A. halophytica showed a similarly high content of GABA to that of a filamentous Arthrospira platensis suggesting the possibility of genetic manipulation of the genes of A. halophytica involved in GABA synthesis to increase GABA yield.




Similar content being viewed by others
References
Bhagwat AA (2003) Regulation of the glutamate-dependent acid-resistance system of diarrheagenic Escherichia coli strains. FEMS Microbiol Lett 227:39–45
Bieleski RL, Turner NA (1966) Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal Biochem 17:278–293
Bolarín MC, Santa-Cruz A, Cayuela E, Pérez-Alfocea F (1995) Short-term solute changes in leaves and roots of cultivated and wild tomato seedlings under salinity. J Plant Physiol 147:463–468
Boonburapong B, Laloknam S, Yamada N, Incharoensakdi A, Takabe T (2012) Sodium-dependent uptake of glutamate by novel ApGltS enhanced growth under salt stress of halotolerant cyanobacterium Aphanothece halophytica. Biosci Biotechnol Biochem 76:1702–1707
Bouche N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9:110–115
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Castanie-Cornet MP, Penfound TA, Smith D, Elliott JF, Foster JW (1999) Control of acid resistance in Escherichia coli. J Bacteriol 181:3525–3535
Eisenhut M, Ruth W, Haimovich M, Bauwe H, Kaplan A, Hagemann M (2008) The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. Proc Natl Acad Sci U S A 105:17199–17204
Henderson JW, Ricker RD, Bidlingmeyer BA, Woodward C (2006) Rapid, accurate, sensitive, and reproducible HPLC analysis of amino acids. Agilent application note 5980-1193EN:1–10
Huang JJ, Kolodny NH, Redfearn JT, Allen MM (2002) The acid stress response of the cyanobacterium Synechocystis sp. strain PCC 6308. Arch Microbiol 177:486–493
Ishitani M, Takabe T, Kojima K, Takabe T (1993) Regulation of glycinebetaine accumulation in the halotolerant cyanobacterium Aphanothece halophytica. Funct Plant Biol 20:693–703
Jiang B, Fu Y, Zhang T (2010) Gamma-aminobutyric acid. In: Bioactive Proteins and Peptides as Functional Foods and Nutraceuticals. Wiley-Blackwell, pp 121–133
Kanwal S, Rastogi R, Incharoensakdi A (2014) Glutamate decarboxylase activity and gamma-aminobutyric acid content in Synechocystis sp. PCC 6803 under osmotic stress and different carbon sources. J Appl Phycol 26:2327–2333
Khetkorn W, Khanna N, Incharoensakdi A, Lindblad P (2013) Metabolic and genetic engineering of cyanobacteria for enhanced hydrogen production. Biofuels 4:535–561
Knoop H, Gründel M, Zilliges Y, Lehmann R, Hoffmann S, Lockau W, Steuer R (2013) flux balance analysis of cyanobacterial metabolism: the metabolic network of Synechocystis sp. PCC 6803. PLoS Comput Biol 9:e1003081
Ma Z, Richard H, Tucker DL, Conway T, Foster JW (2002) Collaborative regulation of Escherichia coli glutamate-dependent acid resistance by two AraC-like regulators, GadX and GadW (YhiW). J Bacteriol 184:7001–7012
Nunnery JK, Mevers E, Gerwick WH (2010) Biologically active secondary metabolites from marine cyanobacteria. Curr Opin Biotechnol 21:787–793
Rastogi RP, Sinha RP, Incharoensakdi A (2014) The cyanotoxin-microcystins: current overview. Rev Environ Sci Biotechnol 13:215–249
Reed RH, Richardson DL, Warr SR, Stewart WD (1984) Carbohydrate accumulation and osmotic stress in cyanobacteria. J Gen Microbiol 130:1–4
Šeputienė V, Daugelavičius A, Sužiedėlis K, Sužiedėlienė E (2006) Acid response of exponentially growing Escherichia coli K-12. Microbiol Res 161:65–74
Shelp BJ, Bown AW, McLean MD (1999) Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4:446–452
Shelp BJ, Bozzo GG, Trobacher CP, Zarei A, Deyman KL, Brikis CJ (2012) Hypothesis/review: contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci 193–194:130–135
Singh S, Kate BN, Banerjee UC (2005) Bioactive compounds from cyanobacteria and microalgae: an overview. Crit Rev Biotechnol 25:73–95
Snedden WA, Arazi T, Fromm H, Shelp BJ (1995) Calcium/calmodulin activation of soybean glutamate decarboxylase. Plant Physiol 108:543–549
Snedden WA, Koutsia N, Baum G, Fromm H (1996) Activation of a recombinant petunia glutamate decarboxylase by calcium/calmodulin or by a monoclonal antibody which recognizes the calmodulin binding domain. J Biol Chem 271:4148–4153
Steinhauser D, Fernie AR, Araujo WL (2012) Unusual cyanobacterial TCA cycles: not broken just different. Trends Plant Sci 17:503–509
Waditee R, Tanaka Y, Aoki K, Hibino T, Jikuya H, Takano J, Takabe T, Takabe T (2003) Isolation and functional characterization of N-methyltransferases that catalyze betaine synthesis from glycine in a halotolerant photosynthetic organism Aphanothece halophytica. J Biol Chem 278:4932–4942
Xing SG, Jun YB, Hau ZW, Liang LY (2007) Higher accumulation of gamma-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. roots. Plant Physiol Biochem 45:560–566
Zhang S, Bryant DA (2011) The tricarboxylic acid cycle in cyanobacteria. Science 334:1551–1553
Acknowledgments
This work was supported by the Office of the Higher Education Commission (OHEC), Thailand, through a grant in the program “Strategic Scholarships for Frontier Research Network for the Ph.D. program, Thai Doctoral degree,” the 90th Anniversary of Chulalongkorn University Ratchadaphiseksomphot Endowment Fund for a Ph.D. scholarship (to BB), and the National Research University project (WCU-013-FW-57) from OHEC (to AI).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boonburapong, B., Laloknam, S. & Incharoensakdi, A. Accumulation of gamma-aminobutyric acid in the halotolerant cyanobacterium Aphanothece halophytica under salt and acid stress. J Appl Phycol 28, 141–148 (2016). https://doi.org/10.1007/s10811-015-0523-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0523-7