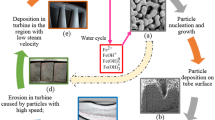
Abstract
Suspension electrolysis is a combined process of chemical and electrochemical reactions. The developed model for a parallel-plate electrochemical reactor is based on mixture model for suspension flow and balance equation for diluted species taking into account the dispersed phase content and ions migration due to the electrolyte current and partial dissolution of suspended particles in the suspension electrolysis. Electrochemical reactions are specified through flux boundary conditions at the electrode/electrolyte interface. The influence of the combined processes is reflected through the distribution of ions concentration profile in liquid phase and current density profile at the electrode surface. Numerical investigation indicates that about 90 % of the iron deposition flux is accommodated by an additional component flux due to the chemical reaction of partial dissolution of α-Fe2O3 particles in suspension electrolysis.

















Similar content being viewed by others
Abbreviations
- \(a\) :
-
Activity (mol l−1)
- \(a_{\text{v}}\) :
-
Interfacial area (m2 m−3)
- \(c\) :
-
Molar concentration (mol m−3)
- \(C_{\text{dl}}\) :
-
Double layer capacitance (F m−2)
- \(d_{\text{p}}\) :
-
Diameter of particle (m)
- \(D\) :
-
Diffusion coefficient (m2 s−1)
- \(D_{\text{a}}\) :
-
Dispersion coefficient (m2 s−1)
- \(E_{\text{cell}}\) :
-
Cell voltage (V)
- \(E_{0}\) :
-
Reversible potential (V)
- \(F\) :
-
Faraday’s constant (96,485 C mol−1)
- \(k\) :
-
Conductivity (Ω−1 m−1)
- \(I\) :
-
Current density (A m−2)
- \(I_{0}\) :
-
Exchange current density (A m−2)
- \(N\) :
-
Molar flow (mol s−1)
- \(n_{{e}}\) :
-
Number of electrons
- \(P\) :
-
Pressure (Pa)
- \(R\) :
-
Ideal gas constant (J mol−1 K−1)
- \(R^{\text{cell}}\) :
-
Ohmic resistance (Ω m2)
- r :
-
Source term (mol m−3 s−1)
- \(S\) :
-
Electrode area (m2)
- \(T\) :
-
Temperature (K)
- \({\mathbf{u}}\) :
-
Velocity vector (m s−1)
- \(V\) :
-
Volume (m3)
- \(x\) :
-
OX co-ordinate
- \(y\) :
-
OY co-ordinate
- \(z\) :
-
OZ co-ordinate
- \(\alpha\) :
-
Dissociation constant
- \(\alpha_{\rm{A}}\) :
-
Anodic charge transfer coefficients
- \(\alpha_{\rm {C}}\) :
-
Cathodic charge transfer coefficients
- \(\alpha_{\rm{G}}\) :
-
Volume fraction of gas phase (m3 m−3)
- \(\beta_{\rm{f}}\) :
-
Mass transfer coefficient (m s−1)
- \(\varepsilon_{\rm{d}}\) :
-
Suspension volume fraction (m3 m−3)
- \(\varGamma\) :
-
Source term (kg m−3 s−1)
- \(\nu\) :
-
Stoichiometry coefficient
- \(\eta\) :
-
Potential difference (V)
- \(\rho\) :
-
Density (kg m−3)
- \(\delta_{\rm{E}}\) :
-
Distance between electrodes (m)
- \(\tau\) :
-
Time (s)
- \(\varphi\) :
-
Potential (V)
- A :
-
Anode electrode
- avg:
-
Averaged
- c :
-
Continuous
- C :
-
Cathode electrode
- cell:
-
Electrochemical reactor
- d :
-
Dispersed
- e :
-
Electrolyte
- eff:
-
Effective
- eq:
-
Equilibrium
- in:
-
Inlet
- L :
-
Liquid
- mol:
-
Molar
- ref:
-
Reference
References
Okada G, Guruswamy V, Bockris JM (1981) J Electrochem Soc 128:2097–2102
Fourcade F, Tzedakis T (2000) J Electroanal Chem 493:20–27
Paramguru RK, Küzeci E, Kammel R (1988) Metall Trans B 19:59–65
Allanore A, Lavelaine H, Birat JP, Valentin G, Lapicque F (2010) J Appl Electrochem 40:1957–1966
Allanore A, Lavelaine H, Valentin G, Birat JP, Lapicque F (2008) J Electrochem Soc 155:E125–E129
Allanore A, Lavelaine H, Valentin G, Birat JP, Lapicque F (2007) J Electrochem Soc 154:E187–E193
Wang CY, Gu WB, Liaw BY (1998) J Electrochem Soc 145:3407–3417
Gu WB, Wang CY (2000) J Electrochem Soc 147:2910–2922
Newman J, Tiedemann W (1975) AIChE J 21:25–41
Haussener S, Xiang C, Spurgeon JM, Ardo S, Lewis NS, Weber AZ (2012) Energy Environ Sci 5:9922–9935
Stefánsson A (2007) Environ Sci Technol 41:6117–6123
Allanore A, Feng J, Lavelaine H, Ogle K (2010) J Electrochem Soc 157:E24–E30
Bockris JM, Huq AS (1956) Proc R Soc Lond A 237(1209):277–296
Balej J (1985) Int J Hydrogen Energy 10:365–374
Hurlen T (1960) Acta Chem Scand 14:1533–1554
Diakonov II, Schott J, Martin F, Harrichourry JC, Escalier J (1999) Geochim Cosmochim Acta 63:2247–2261
Blesa MA, Matijević E (1989) Adv Colloid Interface Sci 29:173–221
Cruz RCD, Reinshagen J, Oberacker R, Segadães AM, Hoffmann MJ (2005) J Colloid Interface Sci 286:579–588
Kastening B, Boinowitz T, Heins M (1997) J Appl Electrochem 27:147–152
Newman JS (1973) Electrochemical systems. Prentice-Hall Inc., Englewood Cliffs
Levin AI (1972) Theoretical fundamentals of electrochemistry. Metallurgiya, Moscow
Purday HFP (1949) An introduction to the mechanics of viscous flow; film lubrication, the flow of heat by conduction and heat transfer by convection. Dover Publications, Mineola
St-Pierre J, Piron DL (1986) J Appl Electrochem 16:447–456
Jupudi R, Zhang H, Zappi G, Bourgeois R (2009) J Comput Multiph Flows 1:341–348
Danilov VA, Tade MO (2009) Int J Hydrogen Energy 34:8998–9006
Bozbiyik B, Danilov VA, Denayer JF (2011) Int J Hydrogen Energy 36:14552–14561
Bansal R (2004) Handbook of engineering electromagnetics. Marcel Dekker, New York
Zemaitis JF, Clark DM, Rafal M, Scrivner NC (2010) Handbook of aqueous electrolyte thermodynamics: Theory & application. Wiley, New York
Bianchi H, Corti HR, Fernandez-Prini R (1994) J Solution Chem 23:1203–1212
Akselrud GA (1970) Mass exchange in the solid-liquid system (Soviet monograph on mass exchange in solid-liquid system covering soluble material extraction and absorption, interphase mass transfer and dissolving process)
Akselrud GA, Bojko AE, Kashcheev AE (1991) J Eng Phys Thermophys 61:98–102
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Charge balance at the electrode/electrolyte interface
The electric potential fields are governed by the charge conservation equations. The charge balance at interface between electron-conducting and ion conducting media is given by [27]
where \(I_{1}\) is the current in electron-conducting media normal to the boundary; \(I_{2}\) is the current in ion –conducting media normal to the boundary; \(I_{\text{s}}\) is the superficial current density; and \(Q\) is the charge. The interfaces between ionic and electronic media behave like a capacitor in which the charge density is a function of potential difference across the double layer. Charge or discharge rate at the electrode–electrolyte double layer can be defined as
where \(\eta\) is the potential differences, \(\eta = \varphi - \varphi_{m}\). For the potential difference at the electrode/electrolyte interface, the following charge conservation equation is valid
Appendix 2: Auxiliary equations for combined processes
The dissociation reaction of α-Fe2O3 in alkaline solution is given by Diakonov et al. [16]
The dissociation constant is defined as
where \(a_{{{\text{Fe(OH)}}_{4}^{ - } }}\) is the activity of \(\rm{Fe(OH)}_{4}^{ - }\) ions and \(a_{{{\text{H}}^{ + } }}\) is the activity of H+ ions. The equilibrium concentration of \(\rm{Fe(OH)}_{4}^{ - }\) ions is a function of H+ ions concentration
Activity of species is associated with the molar concentration \(a = 0.001\gamma c\), where \(\gamma\) is the activity coefficient. The activity coefficient is calculated using NBS smoothed experimental data [28]. The ionic association in NaOH solution is given by
The association constant is
where c NaOH is the molar concentration of NaOH solution; \(\alpha\) is degree of dissociation; and \(\gamma^{{{\text{OH}}^{ - } }}\) is activity coefficient of OH− ions. The constant of association for 50 % NaOH solution is taken from [29]. The degree of dissociation is found from solving (A.2.5) under the given electrolyte concentration. The inlet concentration of OH− and Na+ ions is calculated using the degree of electrolyte dissociation
The inlet concentration of \(\rm{Fe(OH)}_{4}^{ - }\) ions is set equal to the equilibrium concentration \(c_{\text{in}}^{{{\text{Fe(OH)}}_{4}^{ - } }} = 1000 \cdot a^{{{\text{Fe(OH)}}_{4}^{ - } }}\). The next Nernst equation is valid for equilibrium electrochemical reaction (4)
or
Fe(III) ions participate in hydrolysis reactions given by [11, 17]
The hydrolysis constants are defined as follows:
Activity of Fe+3 ions can be expressed as follows:
Taking into account (6), (A.2.11), and (A.2.7), Butler–Volmer equation for the overall cathode electrochemical reaction can be written as
where \(I_{ 0}^{\text{C}}\) is the cathode exchange current density; \(c_{\text{ref}}^{{{\text{Fe(OH)}}_{4}^{ - } }}\) is the reference concentration of \(\rm{Fe(OH)}_{4}^{ - }\) ions; and \(\eta^{\text{C}}\) is the potential difference at the cathode electrode/electrolyte interface.
Hydrogen dissolved in liquid phase is in equilibrium with hydrogen ions at the cathode electrode surface. The equilibrium concentration of hydrogen ions is calculated using Nernst equation defined for equilibrium electrochemical reaction of hydrogen oxidation
Gas content in equilibrium gas–liquid mixture can be calculated from equilibrium flash equation
where \(\gamma_{\rm{G}}\) is the local splitting factor; \(K^{(k)}\) is the distribution of the each components between the vapor and liquid phases, \(K^{(k)} = {{y^{(k)} } \mathord{\left/ {\vphantom {{y^{(k)} } {x^{(k)} }}} \right. \kern-0pt} {x^{(k)} }}\); and \(Z^{(k)}\) is the mixture concentration. The linkage between molar splitting factor and gas volume fraction is given by
Interfacial area of solid particles in suspension is
For suspended particles in liquid flow, mass transfer coefficient in liquid phase is calculated from an empirical correlation [30, 31]
where C is the constant; Sh is the Sherwood number; Sc is the Schmidt number; and Ar is the Archimedes number. The total component balance is written for parallel-plate reactor as follows
Component flow due to the diffusion flow of \(\rm{Fe(OH)}_{4}^{ - }\) ions at the cathode electrode is
Component flow due to the iron deposition at the cathode electrode is
The component flow due to dissolving solid particles in suspension electrolysis is
The main conclusion is that the partial dissolution of solid particles compensates the component flow of iron deposition at the cathode electrode
where S transfer is the contribution of diffusion process, \(S_{\text{transfer}} = {{\left| {M_{\text{transfer}}^{\text{Fe}} } \right|} \mathord{\left/ {\vphantom {{\left| {M_{\text{transfer}}^{\text{Fe}} } \right|} {\left| {M_{\text{deposition}}^{\text{Fe}} } \right|}}} \right. \kern-0pt} {\left| {M_{\text{deposition}}^{\text{Fe}} } \right|}}\) and S dissolving is the contribution of the dissolution process in combined chemical and electrochemical processes, \(S_{\text{dissolving}} = {{\left| {M_{\text{dissolving}}^{\text{Fe}} } \right|} \mathord{\left/ {\vphantom {{\left| {M_{\text{dissolving}}^{\text{Fe}} } \right|} {\left| {M_{\text{deposition}}^{\text{Fe}} } \right|}}} \right. \kern-0pt} {\left| {M_{\text{deposition}}^{\text{Fe}} } \right|}}\).
Rights and permissions
About this article
Cite this article
Danilov, V.A. Numerical investigation of combined chemical and electrochemical processes in Fe2O3 suspension electrolysis. J Appl Electrochem 46, 85–101 (2016). https://doi.org/10.1007/s10800-015-0901-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-015-0901-5