Abstract
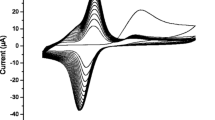
In this work, an electrochemical β-nicotinamide adenine dinucleotide (NADH) sensor based on a carbon paste electrode modified with nickel oxide nanoparticles (NiONPs) was developed. The key highlights of this work are ease of preparation of the NiONPs-modified carbon paste electrode (NiONPs/MCPE), and its high sensitivity to NADH. The electrochemical characterization of NiONPs/MCPEs was performed via cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The electrochemical oxidation response of NADH was investigated by differential pulse voltammetry and chronoamperometry. The results indicated that the electrocatalytic effects of NiONPs on the response current of NADH significantly facilitated the electron transfer and improved the performance of the biosensor. Compared to bare carbon paste electrode (BCPE), the oxidation potential was shifted toward more negative potentials and the oxidation current was increased remarkably. Under optimum conditions, NADH could be detected in the range from 1.0 × 10−4 to 1.0 mmol L−1 with lower detection limit (0.05 μmol L−1). The proposed NADH sensor demonstrated fast and reproducible response. Furthermore, an ethanol biosensor was prepared using NiONPs and NAD+-dependent alcohol dehydrogenase enzyme giving linear responses over the concentration range of 1.6 and 38 mmol L−1 of ethanol.







Similar content being viewed by others
References
Ricci F, Amine A, Moscone D, Palleschi G (2007) A probe for NADH and H2O2 amperometric detection at low applied potential for oxidase and dehydrogenase based biosensor applications. Biosens Bioelectron 22(6):854–862. doi:10.1016/j.bios.2006.03.004
Liu Y, Hou H, You T (2008) Synthesis of carbon nanofibers for mediatorless sensitive detection of NADH. Electroanalysis 20(15):1708–1713. doi:10.1002/elan.200804242
Moiroux J, Elving PJ (1978) Effects of adsorption, electrode material, and operational variables on the oxidation of dihydronicotinamide adenine dinucleotide at carbon electrodes. Anal Chem 50(8):1056–1062. doi:10.1021/ac50030a015
Gligor D, Dilgin Y, Popescu IC, Gorton L (2009) Poly-phenothiazine derivative-modified glassy carbon electrode for NADH electrocatalytic oxidation. Electrochim Acta 54(11):3124–3128. doi:10.1016/j.electacta.2008.11.053
de los Santos Álvarez N, Ortea PM, Pañeda AM, Castañón MJL, Ordieres AJM, Blanco PT (2001) A comparative study of different adenine derivatives for the electrocatalytic oxidation of β-nicotinamide adenine dinucleotide. J Electroanal Chem 502(1–2):109–117. doi:10.1016/S0022-0728(00)00540-4
Zhu L, Yang R, Jiang X, Yang D (2009) Amperometric determination of NADH at a Nile blue/ordered mesoporous carbon composite electrode. Electrochem Commun 11(3):530–533. doi:10.1016/j.elecom.2008.12.045
Mai NN, Liu XY, Zeng XD, Xing L, Wei WZ, Luo SL (2010) Electrocatalytic oxidation of the reduced nicotinamide adenine dinucleotide at carbon ionic liquid electrode modified with polythionine/multi-walled carbon nanotubes composite. Microchim Acta 168(3–4):215–220. doi:10.1007/s00604-009-0285-5
Liu Y, Zhang H-L, Lai G-S, Yu A-M, Huang Y-M, Han D-Y (2010) Amperometric NADH biosensor based on magnetic chitosan microspheres/poly(thionine) modified glassy carbon electrode. Electroanalysis 22(15):1725–1732. doi:10.1002/elan.200900544
Prieto-Simon B, Macanas J, Munoz M, Fabregas E (2007) Evaluation of different mediator-modified screen-printed electrodes used in a flow system as amperometric sensors for NADH. Talanta 71(5):2102–2107. doi:10.1016/j.talanta.2006.09.022
Zheng S, Zhu Y, Krishnaswamy S (2013) Fiber humidity sensors with high sensitivity and selectivity based on interior nanofilm-coated photonic crystal fiber long-period gratings. Sens Actuators B 176(0):264–274. doi:10.1016/j.snb.2012.09.098
Zheng S, Zhu Y, Krishnaswamy S (2012) Nanofilm-coated photonic crystal fiber long-period gratings with modal transition for high chemical sensitivity and selectivity. Proc SPIE 8346, Smart Sensor Phenomena, Technology, Networks, and Systems Integration 2012, 83460D (April 26, 2012). doi:10.1117/12.915050
Tsai Y-C, Huang J-D, Chiu C–C (2007) Amperometric ethanol biosensor based on poly(vinyl alcohol)–multiwalled carbon nanotube–alcohol dehydrogenase biocomposite. Biosens Bioelectron 22(12):3051–3056. doi:10.1016/j.bios.2007.01.005
Guo K, Qian K, Zhang S, Kong J, Yu C, Liu B (2011) Bio-electrocatalysis of NADH and ethanol based on graphene sheets modified electrodes. Talanta 85(2):1174–1179. doi:10.1016/j.talanta.2011.05.038
Arvinte A, Valentini F, Radoi A, Arduini F, Tamburri E, Rotariu L, Palleschi G, Bala C (2007) The NADH electrochemical detection performed at carbon nanofibers modified glassy carbon electrode. Electroanalysis 19(14):1455–1459. doi:10.1002/elan.200703879
Jena BK, Raj CR (2006) Electrochemical biosensor based on integrated assembly of dehydrogenase enzymes and gold nanoparticles. Anal Chem 78(18):6332–6339. doi:10.1021/ac052143f
Teymourian H, Salimi A, Hallaj R (2012) Low potential detection of NADH based on Fe3O4 nanoparticles/multiwalled carbon nanotubes composite: fabrication of integrated dehydrogenase-based lactate biosensor. Biosens Bioelectron 33(1):60–68. doi:10.1016/j.bios.2011.12.031
Curulli A, Valentini F, Padeletti G, Viticoli M, Caschera D, Palleschi G (2005) Smart (Nano) materials: TiO2 nanostructured films to modify electrodes for assembling of new electrochemical probes. Sens Actuators B 111–112:441–449. doi:10.1016/j.snb.2005.03.044
Lata S, Batra B, Karwasra N, Pundir CS (2012) An amperometric H2O2 biosensor based on cytochrome c immobilized onto nickel oxide nanoparticles/carboxylated multiwalled carbon nanotubes/polyaniline modified gold electrode. Process Biochem 47(6):992–998. doi:10.1016/j.procbio.2012.03.018
Hotovy I, Rehacek V, Siciliano P, Capone S, Spiess L (2002) Sensing characteristics of NiO thin films as NO2 gas sensor. Thin Solid Films 418(1):9–15. doi:10.1016/S0040-6090(02)00579-5
Schmid G, Chi LF (1998) Metal clusters and colloids. Adv Mater 10(7):515–526. doi:10.1002/(SICI)1521-4095(199805)10:7<515:AID-ADMA515>3.0.CO;2-Y
Li C, Liu Y, Li L, Du Z, Xu S, Zhang M, Yin X, Wang T (2008) A novel amperometric biosensor based on NiO hollow nanospheres for biosensing glucose. Talanta 77(1):455–459. doi:10.1016/j.talanta.2008.06.048
Ding Y, Liu Y, Zhang L, Wang Y, Bellagamba M, Parisi J, Li CM, Lei Y (2011) Sensitive and selective nonenzymatic glucose detection using functional NiO–Pt hybrid nanofibers. Electrochim Acta 58:209–214. doi:10.1016/j.electacta.2011.09.039
Mu Y, Jia D, He Y, Miao Y, Wu H-L (2011) Nano nickel oxide modified non-enzymatic glucose sensors with enhanced sensitivity through an electrochemical process strategy at high potential. Biosens Bioelectron 26(6):2948–2952. doi:10.1016/j.bios.2010.11.042
Zong S-Z, Cui R-J, Fei L, Li W-W, Ju H-X (2010) Immobilization of myoglobin on NiO nanoparticles matrix for preparation of novel biosensor. Chin J Anal Chem 38(11):1533–1537. doi:10.1016/S1872-2040(09)60074-8
Noorbakhsh A, Salimi A (2011) Development of DNA electrochemical biosensor based on immobilization of ssDNA on the surface of nickel oxide nanoparticles modified glassy carbon electrode. Biosens Bioelectron 30(1):188–196. doi:10.1016/j.bios.2011.09.010
Roushani M, Shamsipur M, Pourmortazavi S (2012) Amperometric detection of glycine, l-serine, and l-alanine using glassy carbon electrode modified by NiO nanoparticles. J Appl Electrochem 42:1–7. doi:10.1007/s10800-012-0475-4
Salimi A, Sharifi E, Noorbakhsh A, Soltanian S (2007) Direct electrochemistry and electrocatalytic activity of catalase immobilized onto electrodeposited nano-scale islands of nickel oxide. Biophys Chem 125(2–3):540–548. doi:10.1016/j.bpc.2006.11.004
Koyuncu D, Erden PE, Pekyardımcı Ş, Kılıç E (2007) A new amperometric carbon paste enzyme electrode for ethanol determination. Anal Lett 40(10):1904–1922. doi:10.1080/00032710701384691
Gilmartin MAT, Hart JP (1995) Sensing with chemically and biologically modified carbon electrodes. A Rev Anal 120(4):1029–1045
Xu L, Du J, Deng Y, He N (2012) Electrochemical detection of E. coli O157:H7 using porous pseudo-carbon paste electrode modified with carboxylic multi-walled carbon nanotubes, glutaraldehyde and 3-aminopropyltriethoxysilane. J Biomed Nanotechnol 8(6):1006–1011. doi:10.1166/jbn.2012.1456
Svancara I, Vytras K, Kalcher K, Walcarius A, Wang J (2009) Carbon paste electrodes in facts, numbers, and notes: a review on the occasion of the 50-years jubilee of carbon paste in electrochemistry and electroanalysis. Electroanalysis 21(1):7–28. doi:10.1002/elan.200804340
Teradal N, Kalanur S, Prashanth SN, Seetharamappa J (2012) Electrochemical investigations of an anticancer drug in the presence of sodium dodecyl sulfate as an enhancing agent at carbon paste electrode. J Appl Electrochem 42(11):917–923. doi:10.1007/s10800-012-0473-6
Santos AS, Freire RS, Kubota LT (2003) Highly stable amperometric biosensor for ethanol based on Meldola’s blue adsorbed on silica gel modified with niobium oxide. J Electroanal Chem 547(2):135–142. doi:10.1016/s0022-0728(03)00186-4
Shang NG, Papakonstantinou P, McMullan M, Chu M, Stamboulis A, Potenza A, Dhesi SS, Marchetto H (2008) Catalyst-free efficient growth, orientation and biosensing properties of multilayer graphene nanoflake films with sharp edge planes. Adv Funct Mater 18(21):3506–3514. doi:10.1002/adfm.200800951
Li F, Li J, Feng Y, Yang L, Du Z (2011) Electrochemical behavior of graphene doped carbon paste electrode and its application for sensitive determination of ascorbic acid. Sens Actuators B 157(1):110–114. doi:10.1016/j.snb.2011.03.033
Zhang Y, Gui Y, Wu X, Feng H, Zhang A, Wang L, Xia T (2009) Preparation of nanostructures NiO and their electrochemical capacitive behaviors. Int J Hydrogen Energy 34(5):2467–2470. doi:10.1016/j.ijhydene.2008.12.078
Zheng Y-Z, Zhang M-L (2007) Preparation and electrochemical properties of nickel oxide by molten-salt synthesis. Mater Lett 61(18):3967–3969. doi:10.1016/j.matlet.2006.12.072
Wang J (2006) Study of electrode reactions and interfacial properties. Analytical electrochemistry. Wiley, Hoboken, pp 29–66. doi:10.1002/0471790303.ch2
F-b Zhang, Y-k Zhou, H-l Li (2004) Nanocrystalline NiO as an electrode material for electrochemical capacitor. Mater Chem Phys 83(2–3):260–264. doi:10.1016/j.matchemphys.2003.09.046
Yao Y, Shiu K–K (2007) Electron-transfer properties of different carbon nanotube materials, and their use in glucose biosensors. Anal Bioanal Chem 387(1):303–309. doi:10.1007/s00216-006-0924-1
Song M-J, Kim J, Lee S, Lee J-H, Lim D, Hwang S, Whang D (2010) Pt-polyaniline nanocomposite on boron-doped diamond electrode for amperometic biosensor with low detection limit. Microchim Acta 171(3–4):249–255. doi:10.1007/s00604-010-0432-z
Adekunle AS, Ozoemena KI (2008) Electron transfer behaviour of single-walled carbon nanotubes electro-decorated with nickel and nickel oxide layers. Electrochim Acta 53(19):5774–5782. doi:10.1016/j.electacta.2008.03.044
Liu X, Li B, Ma M, Zhan G, Liu C, Li C (2012) Amperometric sensing of NADH and ethanol using a hybrid film electrode modified with electrochemically fabricated zirconia nanotubes and poly (acid fuchsin). Microchim Acta 176(1):123–129. doi:10.1007/s00604-011-0701-5
Shan C, Yang H, Han D, Zhang Q, Ivaska A, Niu L (2010) Electrochemical determination of NADH and ethanol based on ionic liquid-functionalized graphene. Biosens Bioelectron 25(6):1504–1508. doi:10.1016/j.bios.2009.11.009
Creanga C, El Murr N (2011) Development of new disposable NADH biosensors based on NADH oxidase. J Electroanal Chem 656(1–2):179–184. doi:10.1016/j.jelechem.2010.11.030
Liu X, Li B, Wang X, Li C (2010) One-step construction of an electrode modified with electrodeposited Au/SiO2 nanoparticles, and its application to the determination of NADH and ethanol. Microchim Acta 171(3–4):399–405. doi:10.1007/s00604-010-0441-y
Yao X, Wang Y, Wen L (2008) Sensitive detection of NADH by ferrocenylalkanethiol functionalized multiwall carbon nanotubes electrodes. Anal Lett 41(7):1236–1247. doi:10.1080/00032710802052692
Lin KC, Yin CY, Chen SM (2012) Electrocatalytic oxidation of NADH based on polyluminol and functionalized multi-walled carbon nanotubes. Analyst 137(6):1378–1383
You C, Xuewu Y, Wang Y, Zhang S, Kong J, Zhao D, Liu B (2009) Electrocatalytic oxidation of NADH based on bicontinuous gyroidal mesoporous carbon with low overpotential. Electrochem Commun 11(1):227–230. doi:10.1016/j.elecom.2008.11.011
Zhu J, Chen X, Yang W (2010) A high performance electrochemical sensor for NADH based on graphite nanosheet modified electrode. Sens Actuators B 150(2):564–568. doi:10.1016/j.snb.2010.08.039
Acknowledgments
The authors gratefully acknowledge the financial support from the Scientific Research Fund of Ankara University (Project No. 12A4240003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aydoğdu, G., Zeybek, D.K., Zeybek, B. et al. Electrochemical sensing of NADH on NiO nanoparticles-modified carbon paste electrode and fabrication of ethanol dehydrogenase-based biosensor. J Appl Electrochem 43, 523–531 (2013). https://doi.org/10.1007/s10800-013-0536-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-013-0536-3