Abstract
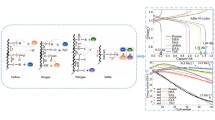
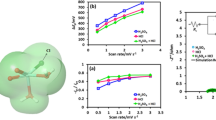
Three electrode materials (glassy carbon, gold, and platinum) were investigated for application in a non-aqueous single-metal redox flow battery based on vanadium (III) acetylacetonate, supported by tetraethylammonium tetrafluoroborate in acetonitrile. Redox couples associated with the one-electron disproportionation of V(acac)3 were observed in voltammetry for each metal tested. An elementary kinetic model was created and used to determine rates for oxidation or reduction of the vanadium complex. The oxidation rates for V(acac)3 were mass-transfer limited on all electrode materials, suggesting reversible kinetics. For the V(acac)3 reduction reaction, exchange-current densities of 1.3, 3.8, and 8.4 A m−2 were observed on glassy carbon, platinum, and gold electrodes, respectively.




Similar content being viewed by others
References
ZBB Energy Corporation (2008) Available via DIALOG. www.zbbenergy.com Cited 15 Dec 2010
Prudent Energy (2009) Available via DIALOG. www.pdenergy.com Cited 15 Dec 2010
Barnartt S, Forejt DA (1964) J Electrochem Soc 111:1201
Sum E, Rychcik M, Skyllas-Kazacos M (1985) J Power Sources 16:85
Sum E, Skyllas-Kazacos M (1985) J Power Sources 15:179
Hagedorn NH, Thaller LH (1981) J Power Sources 8:227
Remick RJ (1984) US Patent 4485154
Zoski C (2007) Handbook of electrochemistry. Elsevier, Amsterdam
Matsuda Y, Takasu Y, Morita M, Tanaka K, Okada M, Matsumura-Inoue T (1985) Denki Kagaku 53:632
Matsuda Y, Tanaka K, Okada M, Takasu Y, Morita M, Matsumura-Inoue T (1988) J Appl Electrochem 18:909
Chakrabarti MH, Dryfe RAW, Roberts EPL (2007) Electrochim Acta 52:2189
Yamamura T, Shiokawa Y, Yamana H, Moriyama H (2002) Electrochim Acta 48:43
Liu Q, Shinkle AA, Li Y, Monroe CW, Thompson LT, Sleightholme AES (2010) Electrochem Comm 12:1634
Sleightholme AES, Shinkle AA, Liu Q, Li Y, Monroe CW, Thompson LT (2011) J Power Sources 196:5742
Liu Q, Sleightholme AES, Shinkle AA, Li Y, Thompson LT (2009) Electrochem Comm 11:2312
Nawi MA, Riechel TL (1981) Inorg Chem 20:1974
Kitamura M, Yamashita K, Imai H (1976) Bull Chem Soc Jpn 49:97
Rychcik M, Skyllas-Kazacos M (1987) J Power Sources 19:45
Hodes G, Manassen J, Cahen D (1980) J Electrochem Soc 127:544
Hollax E, Cheng SH (1985) Carbon 23:655
Lopez-Atalaya M, Codina G, Perez JR, Vazquez JL, Aldaz A, Climent MA (1991) J Power Sources 35:225
Aoki K, Akimoto KT, Matsuda HJ (1984) Electroanal Chem 171:219
Baur JE, Wightman MR (1991) J Electroanal Chem 305:73
Mirkin MV, Bard AJ (1992) Anal Chem 64:2293
Evans DH, Lehmann MW (1999) Acta Chem Scand 53:765
Asselt R, Elsevier CJ, Amatore C, Jutand A (1997) Organometallic 16:317
Norton JD, White HS (1992) J Electroanal Chem 325:341
Bard AJ, Faulkner LR (2001) Electrochemical methods—fundamentals and applications. Wiley, New York
Shinkle AA, Sleightholme AES, Griffith LD, Thompson LT, Monroe CW. J Power Sources Accepted 2010. doi: 10.1016/j.jpowsour.2010.12.096
Nicholson RS, Shain I (1965) Anal Chem 37:1351
Newman J (1970) J Electrochem Soc 17:198
Marcus RA (1963) J Phys Chem 67:853
Fisher AC (1996) Electrode Dynamics. Oxford, UK
Newman J, Thomas-Alyea K (2004) Electrochem Syst. Wiley-Interscience, Hoboken
Zhong S, Skyllas-Kazacos M (1992) J Power Sources 39:1
Acknowledgments
The authors acknowledge financial support from the Advanced Energy for Transportation Technology Program and the Hydrogen Energy Technology Laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shinkle, A.A., Sleightholme, A.E.S., Thompson, L.T. et al. Electrode kinetics in non-aqueous vanadium acetylacetonate redox flow batteries. J Appl Electrochem 41, 1191–1199 (2011). https://doi.org/10.1007/s10800-011-0314-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-011-0314-z