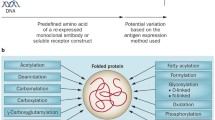
Abstract
Biologic agents have become indispensable in the management of autoimmune disease particularly rheumatological conditions. The lives of countless individuals have been improved following treatment with these drugs. Unfortunately, their cost prohibits more widespread use around the globe. This critical issue has been addressed by the introduction of biosimilars into the market. These therapies have been developed to resemble the originator molecule as closely as possible and to increase competition in the therapy area thus allowing costs to be reduced. Our review is intended to offer an update on biosimilars including logistic considerations on their introduction into routine practice.

Similar content being viewed by others
References
Alvarez A, Ruiz de Castilla E, Flores-Murrieta F, Hughes J, Azevedo V (2014) Recommendations for the regulation of biosimilars and their implementation in Latin America. GaBI J 3:143–148. doi:10.5639/gabij.2014.0303.032
Barile-Fabris L, Irazoque-Palazuelos F, Vasquez R, Vazquez S, Guzman R (2014) Incidence of adverse events in patients treated with intended copies of biologic therapeutic agents in Colombia and Mexico [abstract 1506]. Arthritis Rheumatol 66:S662
Benucci M et al (2016) Safety, efficacy and immunogenicity of switching from innovator to biosimilar infliximab in patients with spondyloarthritis: a 6-month real-life observational study. Immunol Res. doi:10.1007/s12026-016-8843-5
Bhattacharya R, Kamal K (2015) Budget impact analysis of tofacitinib for treatment of rheumatoid arthritis. J Arthritis 4:152. doi:10.4172/2167-7921.1000152
Blackstone EA, Fuhr JP Jr (2007) Biopharmaceuticals: the economic equation. Biotechnol Healthc 4:41–45
Boyce EG, Vyas D, Rogan EL, Valle-Oseguera CS, O’Dell KM (2016) Impact of tofacitinib on patient outcomes in rheumatoid arthritis—review of clinical studies. Patient Relat Outcome Meas 7:1–12. doi:10.2147/prom.s62879
Braun J, Kudrin A (2016) Switching to biosimilar infliximab (CT-P13): evidence of clinical safety, effectiveness and impact on public health. Biologicals 44:257–266. doi:10.1016/j.biologicals.2016.03.006
Brodszky V, Baji P, Balogh O, Pentek M (2014) Budget impact analysis of biosimilar infliximab (CT-P13) for the treatment of rheumatoid arthritis in six Central and Eastern European countries. Eur J Health Econ HEPAC health Econ Prev Care 15(Suppl 1):S65–71. doi:10.1007/s10198-014-0595-3
Claxton L et al (2016) An economic evaluation of tofacitinib treatment in rheumatoid arthritis: modeling the cost of treatment strategies in the United States. J Manag Care Spec Pharm 22(9):1088–1102. doi:10.18553/jmcp.2016.22.9.1088
Cohen S, Genovese M, Choy E, Perez-Ruiz F, Pablos J, Zhang N, Kaur P (2015) Randomized, double-blind, phase 3 study of efficacy and safety of ABP 501 compared with adalimumab in subjects with moderate to severe rheumatoid arthritis [abstract]. Arthritis Rheumatol 67(suppl 10):2443–2444
Declerck P (2012) Biologicals and biosimilars: a review of the science and its implications. GaBI J 1:13–16. doi:10.5639/gabij.2012.0101.005
Derbyshire M (2015) Patent expiry dates for best-selling biologicals. GaBI J 4:178–179. doi:10.5639/gabij.2015.0404.040
Dorner T, Kay J (2015) Biosimilars in rheumatology: current perspectives and lessons learnt. Nat Rev Rheumatol 11:713–724. doi:10.1038/nrrheum.2015.110
Dorner T et al (2013) The role of biosimilars in the treatment of rheumatic diseases. Ann Rheum Dis 72:322–328. doi:10.1136/annrheumdis-2012-202715
Dorner T et al (2016) The changing landscape of biosimilars in rheumatology. Ann Rheum Dis 75:974–982. doi:10.1136/annrheumdis-2016-209166
Elliott MJ et al (1994) Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet (London, England) 344:1105–1110
EMA (2012) Questions and answers on biosimilar medicines (similar biological medicinal products). European medicines agency. http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/12/WC500020062.pdf. Accessed 10 Nov 2016
EMA (2013) Assessment report–CTP-13 (Remsima). European medicines agency. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002576/WC500151486.pdf. Accessed 12 Nove 2016
EMA (2014) Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. European medicines agency. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180219.pdf. Accessed 12 Nov 2016
EMA (2016) Summary of the european public assessment report (EPAR) for benepali. European medicines agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004007/human_med_001944.jsp&mid=WC0b01ac058001d124. Accessed 1 Dec 2016
Emery P et al (2015) A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. doi:10.1136/annrheumdis-2015-207588
Faccin F, Tebbey P, Alexander E, Wang X, Cui L, Albuquerque T (2016) The design of clinical trials to support the switching and alternation of biosimilars. Expert Opin Biol Ther 16:1445–1453. doi:10.1080/14712598.2017.1238454
FDA (2012) FDA approves Xeljanz for rheumatoid arthritis. Food and drug administration. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm327152.htm. Accessed 1 Dec 2016
FDA (2015) Biosimilars: Questions and answers regarding implementation of the biologics price competition and innovation act of 2009: guidance for industry. Food and drug administration. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM444661.pdf Accessed 20 Nov 2016
FDA (2016) FDA approves Amjevita, a biosimilar to Humira. Food and Drug Administration. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed 1 Dec 2016
Flanagan ME et al (2010) Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem 53:8468–8484. doi:10.1021/jm1004286
GBI (2015) Rheumatoid arthritis market to 2020—A crowded market characterized by modest growth. global business intelligence. http://www.gbiresearch.com/report-store/market-reports/therapy-analysis/rheumatoid-arthritis-market-to-2020-a-crowded-market-characterized-by-modest-growth. Accessed 15 Nov 2016
Ghia C, Shah D, Rambhad G, Mubashir A, Upadhaya S (2015) Biologics, biosimilars, intended copies and the era of competitive medicine. Apollo Med 12:103–111. doi:10.1016/j.apme.2015.05.010
Giezen T et al (2016) Roundtable on biosimilars with European regulators and medical societies, Brussels, Belgium, 12 January 2016. GaBI J 5:74–83. doi:10.5639/gabij.2016.0502.019
Jamnitski A et al (2012) Patients non-responding to etanercept obtain lower etanercept concentrations compared with responding patients. Ann Rheum Dis 71:88–91. doi:10.1136/annrheumdis-2011-200184
Kawatkar AA, Jacobsen SJ, Levy GD, Medhekar SS, Venkatasubramaniam KV, Herrinton LJ (2012) Direct medical expenditure associated with rheumatoid arthritis in a nationally representative sample from the medical expenditure panel survey. Arthritis Care Res 64:1649–1656. doi:10.1002/acr.21755
Kim J, Hong J, Kudrin A (2014) 5 year budget impact analysis of biosimilar infliximab for the treatment of rheumatoid arthritis in UK, Italy. France and Germany. Arthritis Rheumatol 11(Suppl):S512 (abstrct 1166)
Kozlowski S, Woodcock J, Midthun K, Sherman RB (2011) Developing the nation’s biosimilars program. N Engl J Med 365:385–388. doi:10.1056/NEJMp1107285
Maini R et al (1999) Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet (London, England) 354:1932–1939
Matucci A, Petroni G, Pratesi S, Nencini F, Maggi E, Vultaggio A (2014) Immunogenicity of biological agents: basic knowledge and clinical implications. Int Trends Immun 2:11–21
McCarthy G, Ebel Bitoun C, Guy H (2013) Introduction of an infliximab biosimilar (CT-P13): a five-year budget impact analysis for the treatment of rheumatoid arthritis in Ireland. Value Health 16:A558. doi:10.1016/j.jval.2013.08.1465
Mellstedt H (2010) The future of biosimilars. Hosp Pharm Eur 49:33–34
Merkesdal S, Ruof J, Huelsemann JL, Mittendorf T, Handelmann S, Mau W, Zeidler H (2005) Indirect cost assessment in patients with rheumatoid arthritis (RA): comparison of data from the health economic patient questionnaire HEQ-RA and insurance claims data. Arthritis Rheum 53:234–240. doi:10.1002/art.21080
Mysler E, Pineda C, Horiuchi T, Singh E, Mahgoub E, Coindreau J, Jacobs I (2016) Clinical and regulatory perspectives on biosimilar therapies and intended copies of biologics in rheumatology. Rheumatol Int 36:613–625. doi:10.1007/s00296-016-3444-0
NICE (2015) Rheumatoid arthritis in adults: management. NICE. https://www.nice.org.uk/guidance/cg79/resources/rheumatoid-arthritis-in-adults-management-975636823525. Accessed 1 Dec 2016
O’Riordan A, Elton J (2016) Healthcare disrupted: next generation business models and strategies. Wiley, New Jersey
Park W et al (2013) A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis 72:1605–1612. doi:10.1136/annrheumdis-2012-203091
Patient Protection and Affordable Care Act (2010). http://www.hhs.gov/healthcare/rights/law/index.html Accessed 23 Nov 2016
Putrik P, Ramiro S, Kvien TK, Sokka T, Uhlig T, Boonen A (2013) Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Are differences related to country’s wealth? Ann Rheum Dis 73:2010–2021. doi:10.1136/annrheumdis-2013-203819
Schiff M et al (2008) Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 67:1096–1103. doi:10.1136/ard.2007.080002
Schneider CK (2013) Biosimilars in rheumatology: the wind of change. Ann Rheum Dis 72:315–318. doi:10.1136/annrheumdis-2012-202941
WHO (2009) Guidelines on evaluation of similar biotherapeutic products (SBPs). World Health Organization. http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf. Accessed 2 Dec 2016
Yoo DH (2014) The rise of biosimilars: potential benefits and drawbacks in rheumatoid arthritis. Expert Rev Clin Immunol 10:981–983. doi:10.1586/1744666x.2014.932690
Yoo DH et al (2013) A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 72:1613–1620. doi:10.1136/annrheumdis-2012-203090
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rischin, A., Östör, A.J.K. Update on biosimilars in rheumatology. Inflammopharmacol 25, 177–184 (2017). https://doi.org/10.1007/s10787-017-0333-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0333-4