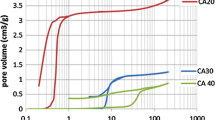
Amorphous carbon samples with a total porosity of about 85% were synthesized via pyrolysis of sol–gel derived resin precursors. Since the pores in the samples investigated have dimensions of a few tens of nanometers only, the gaseous contribution to the thermal conductivity is largely suppressed at ambient pressure. Values for the total thermal conductivity as low as 0.054 W·m−1·K−1 at 300°C are detected. However, the pyrolysis temperature has a great impact on the contribution of the solid backbone to the total thermal conductivity. From the same precursor a series of samples was prepared via pyrolysis at temperatures ranging from 800 to 2500°C. The thermal conductivity of this series of carbons at 300°cC under vacuum increases by a factor of about 8 if the pyrolysis temperature is shifted from 800 to 2500°C. To elucidate the reason for this strong increase, the infrared radiative properties, the electrical conductivity, the macroscopic density, the microcrystallite size, the sound velocity, and the inner surface of the samples were determined. Evaluation of the experimental data yields only a negligible contribution from radiative heat transfer and electronic transport to the total thermal conductivity. The main part of the increasing thermal conductivity therefore has to be attributed to an increasing phonon mean free path in the carbons prepared at higher pyrolysis temperatures. However, the phonon mean free path does not match directly the in-plane microcrystallite size of the amorphous carbon. Rather, the in-plane microcrystallite size represents an upper limit for the phonon mean free path. Hence, the limiting factor for the heat transport via phonons has to be defects swithin the carbon microcrystallites which are partially cured at higher temperatures.
Similar content being viewed by others
References
Pekala R.W. and Kong F.M., “A Synthetic Route to Organic Aerogels – Mechanism, Structure, and Properties,” 2nd Int. Symp. on Aerogels (ISA 2), Montpellier. France, C4, 33–40
Saliger R., Carbon Aerogels for Application in Electrochemical Double Layer Capacitors (Ph.D. Thesis, University of Würzburg, Germany, Report E21 – 1099 −1, 1999).
H. Pröbstle, Kohlenstoffaerogele für den Einsatz in Superkondensatoren (Ph.D. Thesis, University of Würzburg, Germany, Report E21 – 1001 – 1, 2001).
Wiener M., Elektroden aus Kohlenstoffaerogel für PEM-Brennstoffzellen (Diploma Thesis, University of Würzburg, Germany, Report E21 – 1200 – 1 , 2000).
Glora M., Charakterisierung von Gasdiffusionsschichten auf der Basis von Kohlenstoff-Aerogelen für PEM-Brennstoffzellen (Ph.D. Thesis, University of Würzburg, Germany, Report E21 – 0502 – 1, 2002).
Hanzawa Y., Hatori H., Yoshizawa N., Yamada Y., (2002) . Carbon 40:575
Soukup L., Gregora I., Jastrabik L., (1992) . Mater. Sci. Eng. B11:355
Lu X., Nilsson O., Fricke J., Pekala R.W., (1993) . J. Appl. Phys. 73:583
Nilsson O., Bock V., Caps R., and Fricke J., “High Temperature Thermal Properties of Carbon Aerogels,” Thermal Conductivity 22, Proc. Twenty-Second Int. Conf. Therm. Conduct., T.W. Tong, ed. (1994), pp. 878–887.
Bock V., Nilsson O., Blumm J., Fricke J., (1995) . J. Non-Cryst. Solids 185:233
Wiener M., Reichenauer G., Scherb T., Fricke J., (2004) . J. Non-Cryst. Solids 350:126
McCreery R.L., “Carbon Electrodes: Structural Effects on Electron Transfer Kinetics,” in Electroanalytical Chemistry, A Series of Advances, Vol. 17, Bard A.J., ed. (Marcel Dekker, New York, 1991), p. 221.
Knight D.S., White W.B., (1989) . J. Mater. Res. 4:385
Reynolds G.A.M., Fung A.W.P., Wang Z.H., Dresselhaus M.S., Pekala R.W., (1995) . J. Non-Cryst. Solids 188:27
Manara J., Caps R., Rather F., Fricke J., (1999) . Optics Commun. 168:237
Brunauer S., Emmett P.H., Teller E., (1938) . J. Am. Ceram. Soc. 60:309
Nilsson O., Mehling H., Horn R., Fricke J., Hofmann R., Müller S.G., Eckstein R.,Hofmann D., (1997) . High Temps. - High Press. 29:73
Cowan R.D., (1963) . J. Appl. Phys.34:926
National Bureau of Standards, Special Publication 260–89 (1984).
P. G. Klemens, in Thermal Conductivity 1, R. P. Tye, ed. (Academic, London, 1969), p. 1.
Debye P., in Vorträge über die Kinetische Theorie der Materie und der Elektrizität (B. G. Teubner, Berlin, 1914), p. 43.
Reichenauer G., Emmerling A., Fricke J., Pekala R.W., (1998) . J. Non-Cryst. Solids 255:210
Author information
Authors and Affiliations
Corresponding author
Additional information
Paper presented at the Seventeenth European Conference on Thermophysical Properties, September 5–8, 2005, Bratislava, Slovak Republic.
Rights and permissions
About this article
Cite this article
Wiener, M., Reichenauer, G., Hemberger, F. et al. Thermal Conductivity of Carbon Aerogels as a Function of Pyrolysis Temperature. Int J Thermophys 27, 1826–1843 (2006). https://doi.org/10.1007/s10765-006-0086-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-006-0086-6