Abstract
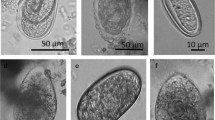
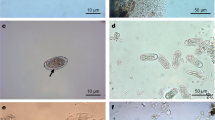
We examined parasite prevalence, abundance of protozoan cysts and helminth eggs in the feces, and number of parasitic taxa in a population of mandrills (Mandrillus sphinx) in semi-free-ranging conditions in their habitat range, with respect to the annual cycle, sex, age, dominance rank, and female reproductive status. We identified 3 taxa of amebic protozoa (Entamoeba coli, E. histolytica/dispar complex, and Endolimax nana), 1 ciliate protozoa (Balantidium coli), and various nematodes. Prevalence ranged from 1 observation in 874 samples for Trichuris and Mammomonogamus (nematodes) to 100% for Entamoeba coli and Endolimax nana. Daily observation, consistency of fecal samples, and periodic veterinary examination indicated that the mandrills were all healthy, suggesting that the presence of intestinal parasites in the provisioned population is well tolerated. Parasite prevalence, abundance in the feces, and number of taxa varied significantly across the annual cycle. Nematode egg prevalence and abundance were lowest during the dry season. We found no sexual difference and no influence of female dominance rank on parasitic infections. Nematode prevalence increased significantly with age in females, but not in males. There was no influence of age on prevalence of other taxa, abundance in the feces, or number of taxa. Abundance of nematode eggs in the feces was higher in pregnant than in lactating or cycling females. However, births are seasonal in the mandrill colony, and pregnant females were present during the months when nematode egg abundance was also higher in males, suggesting that this may be an influence of climatic seasonality in addition to, or instead of, female status.






Similar content being viewed by others
References
Appleton, C. C., & Henzi, S. P. (1993). Environmental correlates of gastrointestinal parasitism in montane and lowland baboons in Natal, South-Africa. International Journal of Primatology, 14, 623–635.
Appleton, C. C., Henzi, S. P., Whiten, A., & Byrne, R. (1986). The gastrointestinal parasites of Papio ursinus from the Drakensberg Mountains, Republic of South-Africa. International Journal of Primatology, 7, 449–456.
Ashford, R. W., Reid, G. D. F., & Wrangham, R. W. (2000). Intestinal parasites of the chimpanzee Pan troglodytes in Kibale Forest, Uganda. Annals of Tropical Medicine and Parasitology, 94, 173–179.
Baskin, G. B. (1993). Pathology of Nonhuman Primates. New Orleans: Tulane Regional Primate Research Centre.
Beagley, K. W., & Gockel, C. M. (2003). Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunology and Medical Microbiology, 38, 13–22.
Beaver, P. C., Jung, R. C., & Cupp, E. W. (1984). Clinical Parasitology. Philadelphia: Lea & Febiger.
Bundy, D. A. P., & Golden, M. H. N. (1987). The impact of host nutrition on gastro-intestinal helminth populations. Parasitology, 95, 623–635.
Charpentier, M., Peignot, P., Hossaert-McKey, M., Gimenez, O., Setchell, J. M., & Wickings, E. J. (2005). Constraints on control: Factors influencing reproductive success in male mandrills (Mandrillus sphinx). Behavioral Ecology, 16, 614–623.
Coltman, D. W., Pilkington, J. G., Smith, J. A., & Pemberton, J. M. (1999). Parasite-mediated selection against inbred Soay sheep in a free-living island population. Evolution, 53, 1259–1267.
Creel, S. (2001). Social dominance and stress hormones. Trends in Ecology and Evolution, 16, 491–497.
Davies, C. R., Ayres, J. M., Dye, C., & Deane, L. M. (1991). Malaria infection rate of Amazonian primates increases with body weight and group size. Functional Ecology, 5, 655–662.
Ettinger, S. J. (1989). Textbook of Veterinary Internal Medicine. Philadelphia: W. B. Saunders.
Fiennes, R. N. T.-W. (1967). Zoonoses of Primates. Ithaca, NY: Cornell University Press.
File, S., & Kessler, M. J. (1989). Parasites of free-ranging Cayo Santiago macaques after 46 years of isolation. American Journal of Primatology, 18, 231–236.
Folstad, I., & Karter, A. J. (1992). Parasites, bright males and the immunocompetence handicap. American Naturalist, 139, 603–622.
Freeland, W. J. (1976). Pathogens and the evolution of primate sociality. Biotropica, 8, 12–24.
Freeman, A. S., Kinsella, J. M., Cipolletta, C., Deem, S. L., & Karesh, W. B. (2004). Endoparasites of western lowland gorillas (Gorilla gorilla gorilla) at Bai Hokou, Central African Republic. Journal of Wildlife Disease, 40, 775–781.
Gillespie, T. R., Greiner, E. C., & Chapman, C. A. (2005). Gastrointestinal parasites of the colobus monkeys of Uganda. Journal of Parasitology, 91, 569–573.
Gulland, F. M. D. (1992). The role of nematode parasites in Soay sheep (Oves aries L.) mortality during a population crash. Parasitology, 105, 493–503.
Hansen, J., & Perry, B. (1994). The epidemiology, diagnosis, and control of helminth parasites of ruminants. Nairobi, Kenya: International Laboratory for Research on Animal Diseases.
Hausfater, G., & Watson, D. F. (1976). Social and reproductive correlates of parasite ova emissions by baboons. Nature, 262, 688–689.
Horii, Y., Imada, I., Yanagida, T., Usui, M., & Mori, A. (1982). Parasite changes and their influence on the body weight of Japanese monkeys (Macaca fuscata fuscata) of the Koshima troop. Primates, 23, 416–431.
Huffman, M. A., Gotoh, S., Turner, L. A., Hamai, M., & Yoshida, K. (1997). Seasonal trends in intestinal nematode infection and medicinal plant use among chimpanzees in the Mahale Mountains, Tanzania. Primates, 38, 111–125.
Klein, S. L. (2004). Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunology, 26, 247–264.
Knezevich, M. (1998). Geophagy as a therapeutic mediator of endoparasitism in a free-ranging group of rhesus macaques (Macaca mulatta). American Journal of Primatology, 44, 71–82.
Koski, K., & Scott, M. (2001). Gastrointestinal nematodes, nutrition, and immunity: Breaking the negative spiral. Annual Review of Nutrition, 21, 297–321.
Lilly, A. A., Mehlman, P. T., & Doran, D. (2002). Intestinal parasites in gorillas, chimpanzees, and humans at Mondika research site, Dzanga-Ndoki National Park, Central African Republic. International Journal of Primatology, 23, 555–573.
Lockborn, T. A. (1948). Balantidium infection associated with diarrhea in primates. Transactions of the Royal Society of Tropical Medicine and Hygiene, 42, 291–293.
Loomis, M. (1983). Hepatic and gastric amebiasis in black and white colobus monkeys. Journal of the American Veterinary Medicine Association, 183, 1188–1191.
McGrew, W. C., Tutin, C. E. G., Collins, D. A., & File, S. K. (1989). Intestinal parasites of sympatric Pan troglodytes and Papio spp. at two sites: Gombe (Tanzania) and Mt Assirik (Senegal). American Journal of Primatology, 17, 147–155.
Milton, K. (1996). Effects of bot fly (Alouattamyia baeri) parasitism on a free-ranging howler monkey (Alouatta palliata) population in Panama. Journal of Zoology, 239, 39–63.
Muehlenbein, M. P. (2005). Parasitological analyses of the male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. American Journal of Primatology, 65, 167–179.
Müller-Graf, C. D. M., Collins, D. A., & Woolhouse, M. E. J. (1996). Intestinal parasite burden in five troops of olive baboons (Papio cynocephalus anubis) in Gombe Stream National Park, Tanzania. Parasitology, 112, 487–497.
Nerrienet, E., Amouretti, X., Muller-Trutwin, M. C., Poaty-Mavoungou, V., Bedjebaga, I., Nguyen, H. T., et al. (1998). Phylogenetic analysis of SIV and STLV type I in mandrills (Mandrillus sphinx): Indications that intracolony transmissions are predominantly the result of male-to-male aggressive contacts. AIDS Research and Human Retroviruses, 14, 785–796.
Nosanchuk, J. S., Wade, S. E., & Landolf, M. (1995). Case report of and description of parasite in Mammomonogamus laryngeus (human syngamosis) infection. Journal of Clinical Microbiology, 33, 998–1000.
Nunn, C. L., & Altizer, S. M. (2005). The global mammal parasite database: An online resource for infectious disease records in wild primates. Evolutionary Anthropology, 14, 1–2.
Nunn, C. L., & Altizer, S. (2006). Infectious diseases in primates: Behavior, ecology and evolution. Oxford, New York: Oxford University Press.
Nunn, C. L., Altizer, S., Jones, K. E., & Sechrest, W. (2003). Comparative tests of parasite species richness in primates. American Naturalist, 162, 597–614.
Paterson, S., Wilson, K., & Pemberton, J. M. (1998). Major histocompatibility complex variation associated with juvenile survival and parasite resistance in a large unmanaged ungulate population (Ovis aries L.). Proceedings of the National Academy of Sciences of the United States of America, 95, 3714–3719.
Robbins, S. L., & Cotran, R. S. (1979). Pathologic Basis of Disease. Philadelphia: W. B. Saunders.
Schad, J., Ganzhorn, J. U., & Sommer, S. (2005). Parasite burden and constitution of major histocompatability complex in the Malagasy mouse lemur, Microcebus murinus. Evolution, 59, 2.
Setchell, J. M., Charpentier, M., Bedjabaga, I-B., Reed, P., Wickings, E. J., & Knapp, L. A. (2006). Secondary sexual characters and female quality in primates. Behavioral Ecology and Sociobiology, 61, 305–315.
Setchell, J. M., Charpentier, M., & Wickings, E. J. (2005). Sexual selection and reproductive careers in mandrills (Mandrillus sphinx). Behavioral Ecology and Sociobiology, 58, 474–485.
Setchell, J. M., & Dixson, A. F. (2002). Developmental variables and dominance rank in male mandrills (Mandrillus sphinx). American Journal of Primatology, 56, 9–25.
Setchell, J. M., Lee, P. C., Wickings, E. J., & Dixson, A. F. (2001). Growth and ontogeny of sexual size dimorphism in the mandrill (Mandrillus sphinx). American Journal of Physical Anthropology, 115, 349–360.
Setchell, J. M., Lee, P. C., Wickings, E. J., & Dixson, A. F. (2002). Reproductive parameters and maternal investment in mandrills (Mandrillus sphinx). International Journal of Primatology, 23, 51–68.
Setchell, J. M., & Wickings, E. J. (2004a). Sequences and timing of dental eruption in mandrills (Mandrillus sphinx). Folia Primatologica, 75, 121–132.
Setchell, J. M., & Wickings, E. J. (2004b). Social and seasonal influences on the reproductive cycle in female mandrills (Mandrillus sphinx). American Journal of Physical Anthropology, 125, 73–84.
Smith, H. A., Jones, T. C., & Hunt, R. D. (1972). Veterinary Pathology. Philadelphia: W. B. Saunders.
Soulsby, E. J. L. (1982). Helminths, arthropods and protozoa of domesticated animals. London: Baillière.
Souquière, S., Bibollet-Ruche, F., Robertson, D. L., Makuwa, M., Apetrei, C., Onanga, R., et al. (2001). Wild Mandrillus sphinx are carriers of two types of lentivirus. Journal of Virology, 75, 7086–7096.
Storey, P. A., Faile, G., Crawley, D., van Oostayen, J. A., Anemana, S., Polderman, A. M., et al. (2001). Ultrasound appearance of preclinical Oesophagostomum bifurcum induced colonic pathology. Gut, 48, 565–566.
Struhsaker, T. T. (1997). Ecology of an African rainforest: Logging in Kibale and the conflict between conservation and exploitation. Gainsville, Florida, USA: Florida University Press.
Stuart, M. D., Greenspan, L. L., Glander, K. E., & Clarke, M. R. (1990). A coprological survey of parasites of wild mantled howling monkeys, Alouatta palliata palliata. Journal of Wildlife Disease, 26, 547–549.
Stuart, M. D., & Strier, K. B. (1995). Primates and parasites: A case for a multidisciplinary approach. International Journal of Primatology, 16, 577–593.
Stuart, M. D., Strier, K. B., & Pierberg, S. M. (1993). A coprological survey of parasites of wild muriquis, Brachyteles arachnoides, and brown howling monkeys, Alouatta fusca. Journal of the Helmointh Society of Washington, 60, 111–115.
Teare, J., & Loomis, M. (1982). Epizootic of balantidiasis in lowland gorillas. Journal of the American Veterinary Medicine Association, 181, 1345–1347.
Vitone, N., Altizer, S. M., & Nunn, C. L. (2004). Body size, diet and sociality influence the species richness of parasitic worms in anthropoid primates. Evolutionary Ecology Research, 6, 1–17.
von Holst, D. (1998). The concept of stress and its relevance for animal behavior. Advances in the Study of Behavior, 27, 1–131.
WHO/PAHO/UNESCO (1997). Report of a consultation of experts on amoebiasis. Weekly Epidemiological Report of the World Health Organisation, 72, 97–99.
Acknowledgments
We thank CIRMF for making this study possible. We thank Oliver Bourry, Paul Bamba, Philippe Engandja, Chabanel Mbou, and other staff of the Primate Centre for their help during the study and Aurelie Gauthier for help with fecal sampling. We thank Michael Huffman and 3 anonymous reviewers for constructive criticism of a previous draft of the manuscript. The CIRMF is financed by the Gabonese government, Total Gabon and the Ministère Français des Affaires Etrangères. This study forms part of a Leverhulme Trust project grant (award no. F/01576/B).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Setchell, J.M., Bedjabaga, IB., Goossens, B. et al. Parasite Prevalence, Abundance, and Diversity in a Semi-free-ranging Colony of Mandrillus sphinx . Int J Primatol 28, 1345–1362 (2007). https://doi.org/10.1007/s10764-007-9225-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-007-9225-6