Abstract
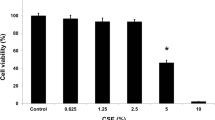
The present study was aimed to investigate the phototherapy effect with low-level laser on human bronchial epithelial cells activated by cigarette smoke extract (CSE). Phototherapy has been reported to actuate positively for controlling the generation/release of anti-inflammatory and pro-inflammatory mediators from different cellular type activated by distinct stimuli. It is not known whether the IL-8 and IL-10 release from CSE-stimulated human bronchial epithelium (BEAS) cells can be influenced by phototherapy. Human bronchial epithelial cell (BEAS) line was cultured in a medium with CSE and irradiated (660 nm) at 9 J. Apoptosis index was standardized with Annexin V and the cellular viability was evaluated by MTT. IL-8, IL-10, cAMP, and NF-κB were measured by ELISA as well as the Sp1, JNK, ERK1/2, and p38MAPK. Phototherapy effect was studied in the presence of mithramycin or the inhibitors of JNK or ERK. The IL-8, cAMP, NF-κB, JNK, p38, and ERK1/2 were downregulated by phototherapy. Both the JNK and the ERK inhibitors potentiated the phototherapy effect on IL-8 as well as on cAMP secretion from BEAS. On the contrary, IL-10 and Sp1 were upregulated by phototherapy. The mithramycin blocked the phototherapy effect on IL-10. The results suggest that phototherapy has a dual effect on BEAS cells because it downregulates the IL-8 secretion by interfering with CSE-mediated signaling pathways, and oppositely upregulates the IL-10 secretion through of Sp1 transcription factor.
Graphical Abstract

The manuscript provides evidence that the phototherapy can interfere with MAPK signaling via cAMP in order to attenuate the IL-8 secretion from CSE-stimulated BEAS. In addition, the present study showed that phototherapy effect is driven to downregulation of the both the IL-8 and the ROS secretion and at the same time the upregulation of IL-10 secretion. Besides it, the increase of Sp-1 transcription factor was crucial for laser effect in upregulating the IL-10 secretion. The dexamethasone corticoid produces a significant inhibitory effect on IL-8 as well as ROS secretion, but on the other hand, the corticoid blocked the IL-10 secretion. Taking it into consideration, it is reasonable to suggest that the beneficial effect of laser therapy on lung diseases involves its action on unbalance between pro-inflammatory and anti-inflammatory mediators secreted by human bronchial epithelial cells through different signaling pathway.












Similar content being viewed by others
References
Chung, K.F., and I.M. Adcock. 2008. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. European Respiratory Journal 31: 1334–1356. https://doi.org/10.1183/09031936.00018908.
Thorley, A.J., and T.D. Tetley. 2007. Pulmonary epithelium, cigarette smoke, and chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease 2: 409–428.
de Vries, M., I.H. Heijink, R. Gras, L.E. den Boef, M. Reinders-Luinge, S.D. Pouwels, M.N. Hylkema, M. van der Toorn, U. Brouwer, A.J. van Oosterhout, and M.C. Nawijn. 2014. Pim1 kinase protects airway epithelial cells from cigarette smoke-induced damage and airway inflammation. American Journal of Physiology. Lung Cellular and Molecular Physiology 307: 240–251.
Abboud, R.T., and S. Vimalanathan. 2008. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. The International Journal of Tuberculosis and Lung Disease 12: 361–367.
Mak, J.C. 2008. Pathogenesis of COPD. Part II. Oxidative-antioxidative imbalance. The International Journal of Tuberculosis and Lung Disease 12: 368–374.
Roth, M. 2008. Pathogenesis of COPD. Part III. Inflammation in COPD. The International Journal of Tuberculosis and Lung Disease 12: 375–380.
Fischer, B.M., E. Pavlisko, and J.A. Voynow. 2011. Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. International Journal of Chronic Obstructive Pulmonary Disease 6: 413–421.
Plataki, M., E. Tzortzaki, P. Rytila, M. Demosthenes, A. Koutsopoulos, and N.M. Siafakas. 2006. Apoptotic mechanisms in the pathogenesis of COPD. International Journal of Chronic Obstructive Pulmonary Disease 1: 161–171.
Ito, K., and P.J. Barnes. 2009. COPD as a disease of accelerated lung aging. Chest. 135: 173–180.
Lee, K.H., C.H. Lee, J. Jeong, A.H. Jang, and C.G. Yoo. 2015. Neutrophil elastase differentially regulates interleukin 8 (IL-8) and vascular endothelial growth factor (VEGF) Production by cigarette smoke extract. The Journal of Biological Chemistry 290: 28438–28445.
O’Donnell, R., D. Breen, S. Wilson, and R. Djukanovic. 2006. Inflammatory cells in the airways in COPD. Thorax. 61: 448–454.
Deslee, G., T.L. Adair-Kirk, T. Betsuyaku, J.C. Woods, C.H. Moore, D.S. Gierada, S.H. Conradi, J.J. Atkinson, H.M. Toennies, J.T. Battaile, D.K. Kobayashi, G.A. Patterson, M.J. Holtzman, and R.A. Pierce. 2010. Cigarette smoke induces nucleic-acid oxidation in lung fibroblasts. American Journal of Respiratory Cell and Molecular Biology 43: 576–584.
Rahman, I., and I.M. Adcock. 2006. Oxidative stress and redox regulation of lung inflammation in COPD. The European Respiratory Journal 28: 219–242.
Milara, J., G. Juan, T. Peiró, A. Serrano, and J. Cortijo. 2012. Neutrophil activation in severe, early-onset COPD patients versus healthy non-smoker subjects in vitro: effects of antioxidant therapy. Respiration. 83 (2): 147–158.
Bjordal, J.M., M. Johnson, V. Iversen, F. Aimbire, and R. Lopes-Martins. 2006. Photoradiation in acute pain: a systemic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomedicine and Laser Surgery 12: 158–168.
Shimizu, N., M. Yamagushi, T. Goseki, Y. Shibata, H. Takiguchi, and T. Iwasawa. 1995. Inhibition of prostaglandin E2 and interleukin-1β production by low power laser irradiation in stretched human periodontal ligament cells. Journal of Dental Research 21: 1382–1388.
Sakurai, Y., M. Yamagushi, and Y. Abiko. 2000. Inhibitory effect of low-level laser irradiation on LPS-stimulated prostaglandin E2 and cyclooxygenase-2 in human gingival fibroblast. European Journal of Oral Sciences 10: 29–34.
Milojevic, M., and V. Kuruc. 2000. Laser biostimulation in the treatment of pleurisy. Medicinski Pregled 18: 516–520.
Landyshev, Iu, N. Avdeeva, N. Goborov, N. Krasavina, G. Tikhonova, and S. Tkacheva. 2002. Efficacy of low intensity laser irradiation and sodium nedocromil in the complex treatment of patients with bronchial asthma. Terapevticheskiĭ Arkhiv 12: 25–28.
Ostronosova, N. 2006. Outpatient use of laser therapy in bronchial asthma. Terapevticheskiĭ Arkhiv 21: 41–44.
Lin, F., S.F. Josephs, D.T. Alexandrescu, F. Ramos, V. Bogin, V. Gammill, C.A. Dasanu, R. De Necochea-Campion, A.N. Patel, E. Carrier, and D.R. Koos. 2010. Lasers, stem cells, and COPD. Journal of Translational Medicine 16: 8–16.
Nikitin, A.V., and S.I. Marks. 2014. The application of chromo- and laserotherapy for the treatment of the patients presenting with chronic obstructive pulmonary disease and concomitant arterial hypertension. Voprosy Kurortologii, Fizioterapii, i Lechebnoĭ Fizicheskoĭ Kultury 4: 3–6.
Aimbire, F., R. Albertine, and R. de Magalhães. 2005. Effect of LLLT Ga-As-Al (685 nm) on LPS-induced inflammation of the airway and lung in rat. Lasers in Medical Science 4: 11–20.
Aimbire, F., R. Albertini, M. Pacheco, F. Aimbire, R. Albertini, M.T. Pacheco, and J.M. Bjordal. 2006. Low-level laser therapy induces dose-dependent reduction of TNF-alpha levels in acute inflammation. Photomedicine and Laser Surgery 10: 33–37.
Mafra de Lima, F., M. Costa, R. Albertini, J. Silva Jr., and F. Aimbire. 2009. Low level laser therapy (LLLT): attenuation of cholinergic hyperreactivity, β2-adrenergic hyporesponsiveness and TNF-α mRNA expression in rat bronchi segments in E. coli lipopolysaccharide-induced airway inflammation by a NF-κB dependent mechanism. Lasers in Surgery and Medicine 3: 68–74.
Aimbire, F., A.P. Ligeiro de Oliveira, R. Albertini, J.C. Corrêa, C.B. Ladeira de Campos, J.P. Lyon, J.A. Silva Jr., and M.S. Costa. 2008. Low-level laser therapy (LLLT) decreases pulmonary microvascular leakage, neutrophil influx and IL-1beta levels in airway and lung from rat subjected to LPS-induced inflammation. Inflammation. 31: 189–197.
Hallstrand, T.S., T.L. Hackett, W.A. Altemeier, G. Matute-Bello, P.M. Hansbro, and D.A. Knight. 2014. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clinical Immunology 151: 1–15.
de Lima, F., A. Villaverde, R. Albertini, A.P. de Oliveira, H. Faria-Neto, and F. Aimbire. 2010. Low-level laser therapy associated to N-acetylcysteine lowers macrophage inflammatory protein-2 (MIP-2) expression and generation of intracellular reactive oxygen species in alveolar macrophage. Photomedicine and Laser Surgery 28: 763–771.
de Lima, F.M., R. Albertini, Y. Dantas, A.L. Maia-Filho, L. Santana Cde, H.C. Castro-Faria-Neto, C. França, A.B. Villaverde, and F. Aimbire. 2013. Low-level laser therapy restores the oxidative stress balance in acute lung injury induced by gut ischemia and reperfusion. Photochemistry and Photobiology 89: 179–188.
de Lima, F., L. Vitoretti, F. Coelho, R. Albertini, A.C. Breithaupt-Faloppa, W. de Lima, and F. Aimbire. 2012. Suppressive effect of low-level laser therapy on tracheal hyperresponsiveness and lung inflammation in rat subjected to intestinal ischemia and reperfusion. Lasers in Medical Science 28: 179–188.
Souza, N.H., P.T. Marcondes, R. Albertini, R.A. Mesquita-Ferrari, K.P. Fernandes, and F. Aimbire. 2014. Low-level laser therapy suppresses the oxidative stress-induced glucocorticoids resistance in U937 cells: relevance to cytokine secretion and histone deacetylase in alveolar macrophages. Journal of Photochemistry and Photobiology 130: 327–336.
Peron, J.P., A.A. de Brito, M. Pelatti, W.N. Brandão, L.B. Vitoretti, F.R. Greiffo, E.C. da Silveira, M.C. Oliveira-Junior, M. Maluf, L. Evangelista, S. Halpern, M.G. Nisenbaum, P. Perin, C.E. Czeresnia, N.O. Câmara, F. Aimbire, P. Vieira Rde, M. Zatz, and A.P. Ligeiro de Oliveira. 2015. Human tubal-derived mesenchymal stromal cells associated with low-level laser therapy significantly reduces cigarette smoke-induced COPD in C57BL/6 mice. PLoS One 10: 532–537.
Ostronosova, N. 2006. Outpatient use of laser therapy in bronchial asthma. Terapevticheskiĭ Arkhiv 78: 41–44.
Kashanskaia, E.P., and A.A. Fedorov. 2009. Low-intensity laser radiation in the combined treatment of patients with chronic obstructive bronchitis. Voprosy Kurortologii, Fizioterapii, i Lechebnoĭ Fizicheskoĭ Kultury 2: 19–22.
Farkhutdinov, U.R., and ShU Farkhutdinov. 2007. Effect of laser radiation on production of reactive oxygen species in the blood of patients with chronic obstructive pulmonary disease. Bulletin of Experimental Biology and Medicine 144: 238–240.
Baker, R., J.R. Pereira da Silva, and J.R. Smith. 2004. The effect of tobacco ingredients on smoke chemistry. part 1: flavorings and additives. Food and Chemical Toxicology 42: 33–37.
Zhao, J., R. Harper, A. Barchowsky, and Y.P. Di. 2007. Identification of multiple MAPK-mediated transcription factors regulated by tobacco smoke in airway epithelial cells. American Journal of Physiology. Lung Cellular and Molecular Physiology 293: 480–490.
Henkel, K.M., K. Frondorf, M.E. Gonzalez-Mejia, A.L. Doseff, and J.G. Cambronero. 2011. IL-8-induced neutrophil chemotaxis is mediated by Janus kinase 3 (JAK3). FEBS Letters 585: 159–166.
Groneberg, D.A., and K.F. Chung. 2004. Models of chronic obstructive pulmonary disease. Respiratory Research 5: 18–21.
Qiu, Y., V. Bandi, R.L. Atmar, K. Hattotuwa, K.K. Guntupalli, and P.K. Jeffery. 2003. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 168: 968–975.
Aimbire, F., F. Santos, R. Albertini, H. Castro-Faria-Neto, J. Mittmann, and C. Pacheco-Soares. 2008. Low-level laser therapy decreases levels of lung neutrophils anti-apoptotic factors by a NF-kB dependent mechanism. International Immunopharmacology 8: 603–605.
Wang, S., S. Kotamraju, E. Konorev, S. Kalivendi, J. Joseph, and B. Kalyanaraman. 2002. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. The Biochemical Journal 367: 729–740.
Lim, W., J. Kim, S. Kim, S. Karna, J. Won, S. Jeon, S. Kim, Y. Choi, H. Choi, and O. Kim. 2013. Modulation of lipopolysaccharide-induced NF-κB signaling pathway by 635 nm irradiation via heat shock protein 27 in human gingival fibroblast cells. Photochemistry and Photobiology 89: 199–207.
Junttila, M.R., S.P. Li, and J. Westermarck. 2008. Phosphatase mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. The FASEB Journal 22: 954–965.
Cossa, G.L., G. Gatti, C. Cassinelli, N. Lanzi, and P. Perego. 2013. Modulation of sensitivity to antitumor agents by targeting the MAPK survival pathway. Current Pharmaceutical Design 19: 883–894.
Renda, T., S. Baraldo, G. Pelaia, E. Bazzan, G. Turato, A. Papi, P. Maestrelli, R. Maselli, A. Vatrella, L.M. Fabbri, R. Zuin, S.A. Marsico, and M. Saetta. 2008. Increased activation of p38 MAPK in COPD. The European Respiratory Journal 31: 62–69.
Rahman, I., and W. MacNee. 1998. Role of transcription factors in inflammatory lung diseases. Thorax. 53: 601–612.
Chung, K.F. 2011. p38 mitogen-activated protein kinase pathways in asthma and COPD. Chest. 139: 1470–1479.
Gaffey, K., S. Reynolds, J. Plumb, M. Kaur, and D. Singh. 2013. Increased phosphorylated p38 mitogen-activated protein kinase in COPD lungs. The European Respiratory Journal 42: 28–41.
Mehra, D., P.M. Geraghty, A.A. Hardigan, and R.A. Foronjy. 2012. Comparison of the inflammatory and proteolytic effects of dung biomass and cigarette smoke exposure in the lung. PLoS One 7: 52889–52933.
Hoffman, E., O. Dittrich-Breiholz, H. Holtmann, and M. Kracht. 2002. Multiple control of interleukin-8 gene expression. Journal of Leukocyte Biology 72: 847–855.
Arndt, P.G., S.K. Young, J.G. Lieber, M.B. Fessler, J.A. Nick, and G.S. Worthen. 2005. Inhibition of c-Jun N-terminal kinase limits lipopolysaccharide-induced pulmonary neutrophil influx. American Journal of Respiratory and Critical Care Medicine 171: 978–986.
Pantano, C., V. Anathy, P. Ranjan, N.H. Heintz, and Y.M. Janssen-Heininger. 2007. Nonphagocytic oxidase 1 causes death in lung epithelial cells via a TNF-RI-JNK signaling axis. American Journal of Respiratory Cell and Molecular Biology 36: 473–479.
Stork, P.J., and J.M. Schmitt. 2002. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends in Cell Biology 12: 258–266.
Holden, N.S., C.F. Rider, M.J. Bell, J. Velayudhan, E.M. King, M. Kaur, M. Salmon, M.A. Giembycz, and R. Newton. 2010. Enhancement of inflammatory mediator release by beta(2)-drenoceptor agonists in airway epithelial cells is reversed by glucocorticoid action. British Journal of Pharmacology 160: 410–420.
Kasama, T., R. Strieter, N. Lukacs, M. Burdick, and S. Kunkel. 1994. Regulation of neutrophil-derived chemokine expression by IL-10. Journal of Immunology 152: 3559–3569.
Pajkrt, D., L. Camoglio, M. Tiel-van Buul, K. de Bruin, D. Cutler, and M. Affrime. 1997. Attenuation of proinflammatory response by recombinant human IL-10 in human endotoxemia: effect of timing of recombinant human IL-10 administration. Journal of Immunology 158: 3971–3977.
Dokka, S., X. Shi, S. Leonard, L. Wang, V. Castranova, and Y. Rojanasakul. 2001. Interleukin-10-mediated inhibition of free radical generation in macrophages. American Journal of Physiology. Lung Cellular and Molecular Physiology 280: 1196–1202.
Deng, J., X. Wang, F. Qian, S. Vogel, L. Xiao, R. Ranjan, H. Park, M. Karpurapu, R. Ye, G. Park, and J. Christman. 2012. Protective role of reactive oxygen species in endotoxin-induced lung inflammation through modulation of IL-10 expression. Journal of Immunology 188: 5734–5740.
Brightbill, H., S. Plevy, R. Modlin, and S. Smale. 2000. A prominent role for Sp1 during lipopolysaccharide-mediated induction of the IL-10 promoter in macrophages. Journal of Immunology 164: 1940–1951.
Tone, M., M. Powell, Y. Tone, S. Thompson, and H. Waldmann. 2000. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. Journal of Immunology 165: 286–291.
Ma, W., W. Lim, K. Gee, S. Aucoin, D. Nandan, M. Kozlowski, F. Diaz-Mitoma, and A. Kumar. 2001. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. The Journal of Biological Chemistry 276: 13664–13674.
Zaman, K., L. Palmer, A. Doctor, J. Hunt, and B. Gaston. 2004. Concentration-dependent effects of endogenous S-nitrosoglutathione on gene regulation by specificity proteins Sp3 and Sp1. The Biochemical Journal 380: 67–74.
Chanteux, H., A. Guisset, C. Pilette, and Y. Sibille. 2007. LPS induces IL-10 production by human alveolar macrophages via MAPKinases- and Sp1-dependent mechanisms. Respiratory Research 8: 71–75.
Henson, P.M., R.W. Vandivier, and I.S. Douglas. 2006. Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proceedings of the American Thoracic Society 3: 713–717.
Funding
This study was sponsored by the Foundation for Research Support of the State of São Paulo (FAPESP) 2012/16498-5.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: FA and APLO. Performed the experiments: AB, TS, KH, MM, AKS, and JLC. Analyzed the data: JLC, APLO, and FA. Contributed reagents/materials/analysis tools: FA, HCFN, RA, and APLO. Wrote the paper: FA and APLO.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 165 kb)
Rights and permissions
About this article
Cite this article
Brito, A., Santos, T., Herculano, K. et al. The MAPKinase Signaling and the Stimulatory Protein-1 (Sp1) Transcription Factor Are Involved in the Phototherapy Effect on Cytokines Secretion from Human Bronchial Epithelial Cells Stimulated with Cigarette Smoke Extract. Inflammation 44, 1643–1661 (2021). https://doi.org/10.1007/s10753-021-01448-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01448-5