Abstract
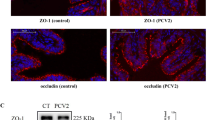
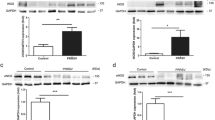
Aquaporins (AQPs) and Na,K-ATPase control water transport across the air space–capillary barrier in the distal lung and play an important role in the formation and resolution of lung edema. Porcine reproductive and respiratory syndrome virus (PRRSV) infection usually causes pulmonary inflammation and edema in the infected pig lungs. To investigate the possibility that PRRSV infection may cause altered expression of AQPs and Na,K-ATPase messenger RNA (mRNA) levels and protein expression of AQP1, AQP5, and Na,K-ATPase in the PRRSV-infected pig lungs were detected. Quantitative real-time PCR (qRT-PCR) analysis showed markedly decreased mRNA levels of AQP1 and AQP5 and Na,K-ATPase in the PRRSV-infected pig lungs compared to those of uninfected pig lungs. Western blot studies also revealed significantly reduced levels of AQP1, AQP5, and Na,K-ATPase proteins in the PRRSV-infected pig lungs. In addition, immunohistochemical (IHC) analysis showed decreased protein expression of AQP1 and AQP5 in the endothelial cells of the capillaries and venules and secretory cells of terminal bronchiole and the alveolar type I cells, respectively. The expression of Na,K-ATPase in the basolateral membrane of alveolar type II cells presented great reduction in the PRRSV-infected pig lungs. To further understand the reduction of these proteins, the ubiquitination of AQP1 and Na,K-ATPase was examined in uninfected and PRRSV-infected pig lungs. The results showed that there is no difference of ubiquitination for these proteins. Thus, our results suggest that PRRSV infection may induce downregulation of these proteins and cause impairment of edema resolution by failed water clearance in the infected pig lungs.







Similar content being viewed by others
References
King, L.S., and P. Agre. 1996. Pathophysiology of the aquaporin water channels. Annual Review of Physiology 58: 619–648. https://doi.org/10.1146/annurev.ph.58.030196.003155.
Agre, P., M. Bonhivers, and M.J. Borgnia. 1998. The aquaporins, blueprints for cellular plumbing systems. The Journal of Biological Chemistry 273 (24): 14659–14662.
King, L.S., D. Kozono, and P. Agre. 2004. From structure to disease: The evolving tale of aquaporin biology. Nature Reviews Molecular Cell Biology 5 (9): 687–698. https://doi.org/10.1038/nrm1469.
Nielsen, S., L.S. King, B.M. Christensen, and P. Agre. 1997. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. The American Journal of Physiology 273 (5 Pt 1): C1549–C1561.
Kreda, S.M., M.C. Gynn, D.A. Fenstermacher, R.C. Boucher, and S.E. Gabriel. 2001. Expression and localization of epithelial aquaporins in the adult human lung. American Journal of Respiratory Cell and Molecular Biology 24 (3): 224–234. https://doi.org/10.1165/ajrcmb.24.3.4367.
King, L.S., and P. Agre. 2001. Man is not a rodent: Aquaporins in the airways. American Journal of Respiratory Cell and Molecular Biology 24 (3): 221–223. https://doi.org/10.1165/ajrcmb.24.3.f202.
Krane, C.M., C.N. Fortner, A.R. Hand, D.W. McGraw, J.N. Lorenz, S.E. Wert, J.E. Towne, R.J. Paul, J.A. Whitsett, and A.G. Menon. 2001. Aquaporin 5-deficient mouse lungs are hyperresponsive to cholinergic stimulation. Proceedings of the National Academy of Sciences of the United States of America 98 (24): 14114–14119. https://doi.org/10.1073/pnas.231273398.
Borok, Z., R.L. Lubman, S.I. Danto, X.L. Zhang, S.M. Zabski, L.S. King, D.M. Lee, P. Agre, and E.D. Crandall. 1998. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. American Journal of Respiratory Cell and Molecular Biology 18 (4): 554–561. https://doi.org/10.1165/ajrcmb.18.4.2838.
Borok, Z., and A.S. Verkman. 2002. Lung edema clearance: 20 years of progress: invited review: role of aquaporin water channels in fluid transport in lung and airways. Journal of Applied Physiology (1985) 93 (6): 2199–2206. https://doi.org/10.1152/japplphysiol.01171.2001.
Dobbs, L.G., R. Gonzalez, M.A. Matthay, E.P. Carter, L. Allen, and A.S. Verkman. 1998. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proceedings of the National Academy of Sciences of the United States of America 95 (6): 2991–2996.
Bai, C., N. Fukuda, Y. Song, T. Ma, M.A. Matthay, and A.S. Verkman. 1999. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. The Journal of Clinical Investigation 103 (4): 555–561. https://doi.org/10.1172/JCI4138.
Matthay, M.A., H.G. Folkesson, and A.S. Verkman. 1996. Salt and water transport across alveolar and distal airway epithelia in the adult lung. The American Journal of Physiology 270 (4 Pt 1): L487–L503.
Sznajder, J.I., P. Factor, and D.H. Ingbar. 2002. Invited review: lung edema clearance: role of Na(+)-K(+)-ATPase. Journal of Applied Physiology (1985) 93 (5): 1860–1866. https://doi.org/10.1152/japplphysiol.00022.2002.
Morgan, S.B., J.P. Frossard, F.J. Pallares, J. Gough, T. Stadejek, S.P. Graham, F. Steinbach, T.W. Drew, and F.J. Salguero. 2016. Pathology and Virus Distribution in the Lung and Lymphoid Tissues of Pigs Experimentally Inoculated with Three Distinct Type 1 PRRS Virus Isolates of Varying Pathogenicity. Transboundary and Emerging Diseases 63 (3): 285–295. https://doi.org/10.1111/tbed.12272.
Tian, K., X. Yu, T. Zhao, Y. Feng, Z. Cao, C. Wang, Y. Hu, et al. 2007. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One 2 (6): e526. https://doi.org/10.1371/journal.pone.0000526.
Bates, J.S., D.B. Petry, J. Eudy, L. Bough, and R.K. Johnson. 2008. Differential expression in lung and bronchial lymph node of pigs with high and low responses to infection with porcine reproductive and respiratory syndrome virus. Journal of Animal Science 86 (12): 3279–3289. https://doi.org/10.2527/jas.2007-0685.
Liu, J., M. Hou, M. Yan, X. Lu, W. Gu, S. Zhang, J. Gao, B. Liu, X. Wu, and G. Liu. 2015. ICAM-1-dependent and ICAM-1-independent neutrophil lung infiltration by porcine reproductive and respiratory syndrome virus infection. American Journal of Physiology. Lung Cellular and Molecular Physiology 309 (3): L226–L236. https://doi.org/10.1152/ajplung.00037.2015.
Schmittgen, T.D., and K.J. Livak. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols 3 (6): 1101–1108.
Ito, J.Y., M. Kawabe, C. Suzukamo, M. Harada, H. Ochiai, and N. Kashiwazaki. 2008. Immunodetection of aquaporin 1 (AQP1) of male and female gametes in pig. Biology of Reproduction: 299–300.
Skowronska, A., P. Mlotkowska, M. Eliszewski, S. Nielsen, and M.T. Skowronski. 2015. Expression of Aquaporin 1, 5 and 9 in the Ovarian Follicles of Cycling and Early Pregnant Pigs. Physiological Research 64 (2): 237–245.
Mimnaugh, E.G., P. Bonvini, and L. Neckers. 1999. The measurement of ubiquitin and ubiquitinated proteins. Electrophoresis 20 (2): 418–428. https://doi.org/10.1002/(SICI)1522-2683(19990201)20:2<418::AID-ELPS418>3.0.CO;2-N.
Effros, R.M., C. Darin, E.R. Jacobs, R.A. Rogers, G. Krenz, and E.E. Schneeberger. 1997. Water transport and the distribution of aquaporin-1 in pulmonary air spaces. Journal of Applied Physiology (1985) 83 (3): 1002–1016.
King, L.S., S. Nielsen, and P. Agre. 1996. Aquaporin-1 water channel protein in lung: ontogeny, steroid-induced expression, and distribution in rat. The Journal of Clinical Investigation 97 (10): 2183–2191. https://doi.org/10.1172/JCI118659.
Towne, J.E., K.S. Harrod, C.M. Krane, and A.G. Menon. 2000. Decreased expression of aquaporin (AQP)1 and AQP5 in mouse lung after acute viral infection. American Journal of Respiratory Cell and Molecular Biology 22 (1): 34–44. https://doi.org/10.1165/ajrcmb.22.1.3818.
Ciechanover, A., A. Orian, and A.L. Schwartz. 2000. Ubiquitin-mediated proteolysis: biological regulation via destruction. BioEssays 22 (5): 442–451. https://doi.org/10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q.
McGrath, M.E. 1999. The lysosomal cysteine proteases. Annual Review of Biophysics and Biomolecular Structure 28: 181–204. https://doi.org/10.1146/annurev.biophys.28.1.181.
Leitch, V., P. Agre, and L.S. King. 2001. Altered ubiquitination and stability of aquaporin-1 in hypertonic stress. Proceedings of the National Academy of Sciences of the United States of America 98 (5): 2894–2898. https://doi.org/10.1073/pnas.041616498.
Hicke, L. 1999. Gettin’ down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends in Cell Biology 9 (3): 107–112.
Walters, E.M., E. Wolf, J.J. Whyte, J. Mao, S. Renner, H. Nagashima, E. Kobayashi, et al. 2012. Completion of the swine genome will simplify the production of swine as a large animal biomedical model. BMC Medical Genomics 5: 55. https://doi.org/10.1186/1755-8794-5-55.
Groenen, M.A.M., A.L. Archibald, H. Uenishi, C.K. Tuggle, Y. Takeuchi, M.F. Rothschild, C. Rogel-Gaillard, et al. 2012. Analyses of pig genomes provide insight into porcine demography and evolution. Nature 491 (7424): 393–398. https://doi.org/10.1038/nature11622.
Verkman, A.S. 2008. From the farm to the lab: the pig as a new model of cystic fibrosis lung disease. American Journal of Physiology. Lung Cellular and Molecular Physiology 295 (2): L238–L239. https://doi.org/10.1152/ajplung.90311.2008.
Rogers, C.S., W.M. Abraham, K.A. Brogden, J.F. Engelhardt, J.T. Fisher, P.B. McCray, G. McLennan, et al. 2008. The porcine lung as a potential model for cystic fibrosis. American Journal of Physiology-Lung Cellular and Molecular Physiology 295 (2): L240–L263. https://doi.org/10.1152/ajplung.90203.2008.
Gushima, Y., K. Ichikado, M. Suga, T. Okamoto, K. Iyonaga, K. Sato, H. Miyakawa, and M. Ando. 2001. Expression of matrix metalloproteinases in pigs with hyperoxia-induced acute lung injury. The European Respiratory Journal 18 (5): 827–837.
Hotchkiss, J.R., M.H. Sanders, G. Clermont, and P.S. Crooke. 2007. Preventing “bored-lung disease” when treating patients with ventilatory failure. Critical Care Medicine 35 (7): 1797–1799. https://doi.org/10.1097/01.Ccm.0000269360.74281.1d.
Sommerer, D., R. Suss, S. Hammerschmidt, H. Wirtz, K. Arnold, and J. Schiller. 2004. Analysis of the phospholipid composition of bronchoalveolar lavage (BAL) fluid from man and minipig by MALDI-TOF mass spectrometry in combination with TLC. Journal of Pharmaceutical and Biomedical Analysis 35 (1): 199–206. https://doi.org/10.1016/j.jpba.2003.12.016.
Thanawongnuwech, R., B. Thacker, P. Halbur, and E.L. Thacker. 2004. Increased production of proinflammatory cytokines following infection with porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae. Clinical and Diagnostic Laboratory Immunology 11 (5): 901–908. https://doi.org/10.1128/CDLI.11.5.901-908.2004.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) Grants 31372418 (G. Liu) and 31460563 (J. Zhang), Huazhong Agricultural University Scientific and Technology Self-Innovation Foundation Program 2012RC011 (G. Liu), and the 948 Project of Chinese Ministry of Agriculture (2015-Z33, GL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal experiments were approved by the Animal Care and Use Committee of Hubei Province, China, in accordance with guidelines developed by the China Council on Animal Care and protocol.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, J., Yan, M., Gu, W. et al. Downregulation of Aquaporins (AQP1 and AQP5) and Na,K-ATPase in Porcine Reproductive and Respiratory Syndrome Virus-Infected Pig Lungs. Inflammation 41, 1104–1114 (2018). https://doi.org/10.1007/s10753-018-0762-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0762-2