Abstract
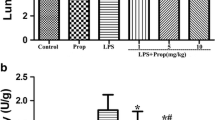
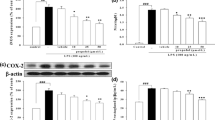
Propofol, an intravenous anesthetic agent widely used in clinical practice, is the preferred anesthetic for asthmatic patients. This study was designed to determine the protective effect and underlying mechanisms of propofol on airway inflammation in a mast cell-dependent mouse model of allergic asthma. Mice were sensitized by ovalbumin (OVA) without alum and challenged with OVA three times. Propofol was given intraperitoneally 0.5 h prior to OVA challenge. The inflammatory cell count and production of cytokines in the bronchoalveolar lavage fluid (BALF) were detected. The changes of lung histology and key molecules of the toll-like receptor 4 (TLR4)/reactive oxygen species (ROS)/NF-κB signaling pathway were also measured. The results showed that propofol significantly decreased the number of eosinophils and the levels of IL-4, IL-5, IL-6, IL-13, and TNF-α in BALF. Furthermore, propofol significantly attenuated airway inflammation, as characterized by fewer infiltrating inflammatory cells and decreased mucus production and goblet cell hyperplasia. Meanwhile, the expression of TLR4, and its downstream signaling adaptor molecules—–myeloid differentiation factor 88 (MyD88) and NF-κB, were inhibited by propofol. The hydrogen peroxide and methane dicarboxylic aldehyde levels were decreased by propofol, and the superoxide dismutase activity was increased in propofol treatment group. These findings indicate that propofol may attenuate airway inflammation by inhibiting the TLR4/MyD88/ROS/NF-κB signaling pathway in a mast cell-dependent mouse model of allergic asthma.







Similar content being viewed by others
References
Kim, H.Y., R.H. DeKruyff, and D.T. Umetsu. 2010. The many paths to asthma: Phenotype shaped by innate and adaptive immunity. Nature Immunology 11: 577–584.
Hamid, Q., and M. Tulic. 2009. Immunobiology of asthma. Annual Review of Physiology 71: 489–507.
Anandan, C., U. Nurmatov, O.C. van Schayck, and A. Sheikh. 2010. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy 65: 152–167.
Ie, K., A. Yoshizawa, S. Hirano, S. Izumi, M. Hojo, H. Sugiyama, N. Kobayasi, et al. 2010. A survey of perioperative asthmatic attack among patients with bronchial asthma underwent general anesthesia. Arerugī 59: 831–838.
Woods, B.D., and R.N. Sladen. 2009. Perioperative considerations for the patient with asthma and bronchospasm. British Journal of Anaesthesia 103: i57–i65.
Chan, A.L., M.M. Juarez, N. Gidwani, and T.E. Albertson. 2015. Management of critical asthma syndrome during pregnancy. Clinical Reviews in Allergy and Immunology 48: 45–53.
Grim, K.J., A.J. Abcejo, A. Barnes, V. Sathish, D.F. Smelter, G.C. Ford, M.A. Thompson, Y.S. Prakash, and C.M. Pabelick. 2012. Caveolae and propofol effects on airway smooth muscle. British Journal of Anaesthesia 109: 444–453.
Meng, J., X. Xin, Z. Liu, H. Li, B. Huang, Y. Huang, and J. Zhao. 2016. Propofol inhibits T-helper cell type-2 differentiation by inducing apoptosis via activating gamma-aminobutyric acid receptor. The Journal of Surgical Research 206: 442–450.
Andersson, C., E. Tufvesson, Z. Diamant, and L. Bjermer. 2016. Revisiting the role of the mast cell in asthma. Current Opinion in Pulmonary Medicine 22: 10–17.
Zuo, L., K. Lucas, C.A. Fortuna, C.C. Chuang, and T.M. Best. 2015. Molecular regulation of toll-like receptors in asthma and COPD. Frontiers in Physiology 6: 312.
Kawai, T., and S. Akira. 2010. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nature Immunology 11: 373–384.
Yamashita, M., and T. Nakayama. 2008. Progress in allergy signal research on mast cells: Regulation of allergic airway inflammation through toll-like receptor 4-mediated modification of mast cell function. Journal of Pharmacological Sciences 106: 332–335.
Nigo, Y.I., M. Yamashita, K. Hirahara, R. Shinnakasu, M. Inami, M. Kimura, A. Hasegawa, Y. Kohno, and T. Nakayama. 2006. Regulation of allergic airway inflammation through toll-like receptor 4-mediated modification of mast cell function. Proceedings of the National Academy of Sciences of the United States of America 103: 2286–2291.
Lee, A.J., M. Ro, K.J. Cho, and J.H. Kim. 2017. Lipopolysaccharide/TLR4 stimulates IL-13 production through a MyD88-BLT2-linked cascade in mast cells, potentially contributing to the allergic response. Vol. 199, 409–417.
Schnare, M., G.M. Barton, A.C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nature Immunology 2: 947–950.
Comhair, S.A., and S.C. Erzurum. 2010. Redox control of asthma: Molecular mechanisms and therapeutic opportunities. Antioxidants & Redox Signaling 12: 93–124.
Nadeem, A., N. Siddiqui, N.O. Alharbi, and M.M. Alharbi. 2014. Airway and systemic oxidant-antioxidant dysregulation in asthma: A possible scenario of oxidants spill over from lung into blood. Pulmonary Pharmacology & Therapeutics 29: 31–40.
Edwards, M.R., N.W. Bartlett, D. Clarke, M. Birrell, M. Belvisi, and S.L. Johnston. 2009. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacology & Therapeutics 121: 1–13.
Schuliga, M. 2015. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules 5: 1266–1283.
Zhou, C.H., Y.Z. Zhu, P.P. Zhao, C.M. Xu, M.X. Zhang, H. Huang, J. Li, L. Liu, and Y.Q. Wu. 2015. Propofol inhibits lipopolysaccharide-induced inflammatory responses in spinal astrocytes via the toll-like receptor 4/MyD88-dependent nuclear factor-kappaB, extracellular signal-regulated protein kinases1/2, and p38 mitogen-activated protein kinase pathways. Anesthesia and Analgesia 120: 1361–1368.
Ulbrich, F., L. Eisert, H. Buerkle, U. Goebel, and N. Schallner. 2016. Propofol, but not ketamine or midazolam, exerts neuroprotection after ischaemic injury by inhibition of toll-like receptor 4 and nuclear factor kappa-light-chain-enhancer of activated B-cell signalling: A combined in vitro and animal study. European Journal of Anaesthesiology 33: 670–680.
Price, M.M., C.A. Oskeritzian, Y.T. Falanga, K.B. Harikumar, J.C. Allegood, S.E. Alvarez, D. Conrad, J.J. Ryan, S. Milstien, and S. Spiegel. 2013. A specific sphingosine kinase 1 inhibitor attenuates airway hyperresponsiveness and inflammation in a mast cell-dependent murine model of allergic asthma. J Allergy Clin Immunol 131 (e501): 501–511.
Nakae, S., C. Lunderius, L.H. Ho, B. Schafer, M. Tsai, and S.J. Galli. 2007. TNF can contribute to multiple features of ovalbumin-induced allergic inflammation of the airways in mice. The Journal of Allergy and Clinical Immunology 119: 680–686.
Williams, C.M., and S.J. Galli. 2000. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. The Journal of Experimental Medicine 192: 455–462.
Myou, S., A.R. Leff, S. Myo, E. Boetticher, J. Tong, A.Y. Meliton, J. Liu, N.M. Munoz, and X. Zhu. 2003. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. The Journal of Experimental Medicine 198: 1573–1582.
Padrid, P., S. Snook, T. Finucane, P. Shiue, P. Cozzi, J. Solway, and A.R. Leff. 1995. Persistent airway hyperresponsiveness and histologic alterations after chronic antigen challenge in cats. American Journal of Respiratory and Critical Care Medicine 151: 184–193.
Burburan, S.M., D.G. Xisto, and P.R. Rocco. 2007. Anaesthetic management in asthma. Minerva Anestesiologica 73: 357–365.
Kuperman, D.A., X. Huang, L.L. Koth, G.H. Chang, G.M. Dolganov, Z. Zhu, J.A. Elias, D. Sheppard, and D.J. Erle. 2002. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nature Medicine 8: 885–889.
Luttmann, W., T. Matthiesen, H. Matthys, and J. C. Virchow, Jr. 1999. Synergistic effects of interleukin-4 or interleukin-13 and tumor necrosis factor-alpha on eosinophil activation in vitro. American Journal of Respiratory Cell and Molecular Biology 20:474–480.
Shakoory, B., S.M. Fitzgerald, S.A. Lee, D.S. Chi, and G. Krishnaswamy. 2004. The role of human mast cell-derived cytokines in eosinophil biology. Journal of Interferon & Cytokine Research 24: 271–281.
O’Neill, L.A. 2008. Primer: Toll-like receptor signaling pathways—what do rheumatologists need to know? Nature Clinical Practice. Rheumatology 4: 319–327.
Ye, H.H., K.J. Wu, S.J. Fei, X.W. Zhang, H.X. Liu, J.L. Zhang, and Y.M. Zhang. 2013. Propofol participates in gastric mucosal protection through inhibiting the toll-like receptor-4/nuclear factor kappa-B signaling pathway. Clinics and Research in Hepatology and Gastroenterology 37: e3–15.
Dikmen, B., H. Yagmurdur, T. Akgul, M. Astarci, H. Ustun, and C. Germiyanoglu. 2010. Preventive effects of propofol and ketamine on renal injury in unilateral ureteral obstruction. Journal of Anesthesia 24: 73–80.
Bandeiras, C., A.P. Serro, K. Luzyanin, A. Fernandes, and B. Saramago. 2013. Anesthetics interacting with lipid rafts. European Journal of Pharmaceutical Sciences 48: 153–165.
Wu, G.J., T.L. Chen, C.C. Chang, and R.M. Chen. 2009. Propofol suppresses tumor necrosis factor-alpha biosynthesis in lipopolysaccharide-stimulated macrophages possibly through downregulation of nuclear factor-kappa B-mediated toll-like receptor 4 gene expression. Chemico-Biological Interactions 180: 465–471.
Gan, X., D. Xing, G. Su, S. Li, C. Luo, M.G. Irwin, Z. Xia, H. Li, and Z. Hei. 2015. Propofol attenuates small intestinal ischemia reperfusion injury through inhibiting NADPH oxidase mediated mast cell activation. Oxidative Medicine and Cellular Longevity 2015: 167014.
Zhao, W., S. Zhou, W. Yao, X. Gan, G. Su, D. Yuan, and Z. Hei. 2014. Propofol prevents lung injury after intestinal ischemia–reperfusion by inhibiting the interaction between mast cell activation and oxidative stress. Life Sciences 108: 80–87.
Inoue, T., Y. Suzuki, T. Yoshimaru, and C. Ra. 2008. Reactive oxygen species produced up- or downstream of calcium influx regulate proinflammatory mediator release from mast cells: Role of NADPH oxidase and mitochondria. Biochimica et Biophysica Acta 1783: 789–802.
Kuehn, H.S., E.J. Swindle, M.S. Kim, M.A. Beaven, D.D. Metcalfe, and A.M. Gilfillan. 2008. The phosphoinositide 3-kinase-dependent activation of Btk is required for optimal eicosanoid production and generation of reactive oxygen species in antigen-stimulated mast cells. Journal of Immunology 181: 7706–7712.
Cho, K.J., J.M. Seo, M.G. Lee, and J.H. Kim. 2010. BLT2 is upregulated in allergen-stimulated mast cells and mediates the synthesis of Th2 cytokines. Journal of Immunology 185: 6329–6337.
Tully, J.E., S.M. Hoffman, K.G. Lahue, J.D. Nolin, V. Anathy, L.K. Lundblad, N. Daphtary, et al. 2013. Epithelial NF-kappaB orchestrates house dust mite-induced airway inflammation, hyperresponsiveness, and fibrotic remodeling. Journal of Immunology 191: 5811–5821.
Mitchell, S., J. Vargas, and A. Hoffmann. 2016. Signaling via the NFkappaB system. Wiley Interdisciplinary Reviews. Systems Biology and Medicine 8: 227–241.
Shin, I.W., I.S. Jang, S.H. Lee, J.S. Baik, K.E. Park, J.T. Sohn, H.K. Lee, and Y.K. Chung. 2010. Propofol has delayed myocardial protective effects after a regional ischemia/reperfusion injury in an in vivo rat heart model. Korean J Anesthesiol 58: 378–382.
Yuzbasioglu, M.F., A. Aykas, E.B. Kurutas, and T. Sahinkanat. 2010. Protective effects of propofol against ischemia/reperfusion injury in rat kidneys. Renal Failure 32: 578–583.
Shibuya, K., T. Ishiyama, M. Ichikawa, H. Sato, K. Okuyama, D.I. Sessler, and T. Matsukawa. 2009. The direct effects of propofol on pial microvessels in rabbits. Journal of Neurosurgical Anesthesiology 21: 40–46.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81250026), Graduate Student Innovation Foundation of Peking Union Medical College (201510020202), and Initial Scientific Research foundation for talent introduction of China–Japan Friendship Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, HY., Meng, JX., Liu, Z. et al. Propofol Attenuates Airway Inflammation in a Mast Cell-Dependent Mouse Model of Allergic Asthma by Inhibiting the Toll-like Receptor 4/Reactive Oxygen Species/Nuclear Factor κB Signaling Pathway. Inflammation 41, 914–923 (2018). https://doi.org/10.1007/s10753-018-0746-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0746-2