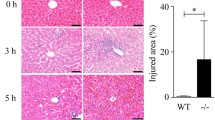
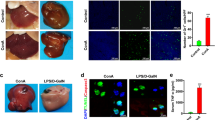
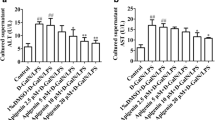
Abstract
Fulminant hepatic failure (FHF) is a life-threatening clinical syndrome results in massive inflammation and hepatocyte death. Necroptosis is a regulated form of necrotic cell death that is emerging as a crucial control point for inflammatory diseases. The kinases receptor interacting protein (RIP) 1 and RIP3 are known as key modulators of necroptosis. In this study, we investigated the impact of necroptosis in the pathogenesis of FHF and molecular mechanisms, particularly its linkage to damage-associated molecular pattern (DAMP)-mediated pattern recognition receptor (PRR) signaling pathways. Male C57BL/6 mice were given an intraperitoneal injection of necrostatin-1 (Nec-1, RIP1 inhibitor; 1.8 mg/kg; dissolved in 2% dimethyl sulfoxide in phosphate-buffered saline) 1 h before receiving d-galactosamine (GalN; 800 mg/kg)/lipopolysaccharide (LPS; 40 μg/kg). Hepatic RIP1, RIP3 protein expression, their phosphorylation, and RIP1/RIP3 complex formation upregulated in the GalN/LPS group were attenuated by Nec-1. Nec-1 markedly reduced the increases in mortality and serum alanine aminotransferase activity induced by GalN/LPS. Increased serum high mobility group box 1 (HMGB1) and interleukin (IL)-33 release, HMGB1-toll-like receptor 4 and HMGB1-receptor for advanced glycation end products (RAGE) interaction, and nuclear protein expressions of NF-κB and early growth response protein-1 (egr-1) were attenuated by Nec-1. Our finding suggests that necroptosis is responsible for GalN/LPS-induced liver injury through DAMP-activated PRR signaling.





Similar content being viewed by others
References
Micheau, O., and J. Tschopp. 2003. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114 (2): 181–190.
Chan, F. 2012. Fueling the flames: mammalian programmed necrosis in inflammatory diseases. Cold Spring Harbor Perspectives in Biology 4 (11): a008805. doi:10.1101/cshperspect.a008805.
Vandenabeele, P., L. Galluzzi, T. Vanden Berghe, and G. Kroemer. 2010. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature Reviews. Molecular Cell Biology 11 (10): 700–714. doi:10.1038/nrm2970.
Degterev, A., Z. Huang, M. Boyce, Y. Li, P. Jagtap, N. Mizushima, G.D. Cuny, T.J. Mitchison, M.A. Moskowitz, and J. Yuan. 2005. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature Chemical Biology 1 (2): 112–119. doi:10.1038/nchembio711.
Li, Y., X. Yang, C. Ma, J. Qiao, and C. Zhang. 2008. Necroptosis contributes to the NMDA-induced excitotoxicity in rat’s cultured cortical neurons. Neuroscience Letters 447 (2–3): 120–123. doi:10.1016/j.neulet.2008.08.037.
Oerlemans, M.I., J. Liu, F. Arslan, K. den Ouden, B.J. van Middelaar, P.A. Doevendans, and J.P. Sluijter. 2012. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Research in Cardiology 107 (4): 270–278. doi:10.1007/s00395-012-0270-8.
Xu, X., C.C. Chua, J. Kong, R.M. Kostrzewa, U. Kumaraguru, R.C. Hamdy, and B.H. Chua. 2007. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. Journal of Neurochemistry 103 (5): 2004–2014. doi:10.1111/j.1471- 4159.2007.04884.x.
Jouan-Lanhouet, S., M.I. Arshad, C. Piquet-Pellorce, C. Martin-Chouly, G. Le Moigne-Muller, F. Van Herreweghe, N. Takahashi, et al. 2012. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death and Differentiation 19: 2003–2014. doi:10.1038/cdd.2012.90.
Zhou, Y., W. Dai, C. Lin, F. Wang, L. He, M. Shen, P. Chen, et al. 2013. Protective effects of necrostatin-1 against concanavalin A-induced acute hepatic injury in mice. Mediators of Inflammation 2013: 706156. doi:10.1155/2013/706156.
Zhang, Y.F., W. He, C. Zhang, X.J. Liu, Y. Lu, H. Wang, Z.H. Zhang, X. Chen, and D.X. Xu. 2014. Role of receptor interacting protein (RIP)1 on apoptosis-inducing factor-mediated necroptosis during acetaminophen-evoked acute liver failure in mice. Toxicology Letters 225 (3): 445–453. doi:10.1016/j.toxlet.2014.01.005.
Takemoto, K., E. Hatano, K. Iwaisako, M. Takeiri, N. Noma, S. Ohmae, K. Toriguchi, et al. 2014. Necrostatin-1 protects against reactive oxygen species (ROS)-induced hepatotoxicity in acetaminophen-induced acute liver failure. FEBS Open Bio 4 (1): 777–787. doi:10.1016/j.fob.2014.08.007.
Ramachandran, A., M.R. McGill, Y. Xie, H.M. Ni, W.X. Ding, and H. Jaeschke. 2013. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology 58 (6): 2099–2108. doi:10.1002/hep.26547.
Roychowdhury, S., M.R. McMullen, S.G. Pisano, X. Liu, and L.E. Nagy. 2013. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology 57 (5): 1773–1783. doi:10.1002/hep.26200.
Lau, A., S. Wang, J. Jiang, A. Haig, A. Pavlosky, A. Linkermann, Z.X. Zhang, and A.M. Jevnikar. 2013. RIPK3-mediated necroptosis promotes donor kidney inflammatory injury and reduces allograft survival. American Journal of Transplantation 13 (11): 2805–2818. doi:10.1111/ajt.12447.
Murakami, Y., H. Matsumoto, M. Roh, A. Giani, K. Kataoka, Y. Morizane, M. Kayama, et al. 2014. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death and Differentiation 21 (2): 270–277. doi:10.1038/cdd.2013.109.
He, S., Y. Liang, F. Shao, and X. Wang. 2011. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proceedings of the National Academy of Sciences of the United States of America 108 (50): 20054–20059. doi:10.1073/pnas.1116302108.
Kaczmarek, A., P. Vandenabeele, and D.V. Krysko. 2013. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38 (2): 209–223. doi:10.1016/j.immuni.2013.02.003.
Zeng, S., H. Dun, N. Ippagunta, R. Rosario, Q.Y. Zhang, J. Lefkowitch, S.F. Yan, A.M. Schmidt, and J.C. Emond. 2009. Receptor for advanced glycation end product (RAGE)-dependent modulation of early growth response-1 in hepatic ischemia/reperfusion injury. Journal of Hepatology 50 (5): 929–936. doi:10.1016/j.jhep.2008.11.022.
Mocarski, E.S., H. Guo, and W.J. Kaiser. 2015. Necroptosis: the Trojan horse in cell autonomous antiviral host defense. Virology 479–480: 160–166. doi:10.1016/j.virol.2015.03.016.
Wu, J., Z. Huang, J. Ren, Z. Zhang, P. He, Y. Li, J. Ma, et al. 2013. MLKL knockout mice demonstrate the indispensable role of MLKL in necroptosis. Cell Research 23 (8): 994–1006. doi:10.1038/cr.2013.91.
Kim, S.J., J.K. Kim, D.U. Lee, J.H. Kwak, and S.M. Lee. 2010. Genipin protects lipopolysaccharide-induced apoptotic liver damage in D-galactosamine-sensitized mice. European Journal of Pharmacology 635 (1–3): 188–193. doi:10.1016/j.ejphar.2010.03.007.
Murphy, J. M., and J. E. Vince. 2015. Post-translational control of RIPK3 and MLKL mediated necroptotic cell death. F1000Research 4. doi:10.12688/f1000research.7046.1.
Remijsen, Q., V. Goossens, S. Grootjans, C. Van den Haute, N. Vanlangenakker, Y. Dondelinger, R. Roelandt, et al. 2014. Depletion of RIPK3 or MLKL blocks TNF-driven necroptosis and switches towards a delayed RIPK1 kinase-dependent apoptosis. Cell Death and Disease 5: e1004. doi:10.1038/cddis.2013.531.
Murphy, J.M., P.E. Czabotar, J.M. Hildebrand, I.S. Lucet, J.G. Zhang, S. Alvarez-Diaz, R. Lewis, et al. 2016. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39 (3): 443–453. doi:10.1016/j.immuni.2013.06.018.
Afonso, M.B., P.M. Rodrigues, T. Carvalho, M. Caridade, P. Borralho, H. Cortez-Pinto, R.E. Castro, and C.M.P. Rodrigues. 2015. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clinical Science 129 (8): 721–739. doi:10.1042/CS20140732.
Morikawa, A., T. Sugiyama, Y. Kato, N. Koide, G.Z. Jiang, K. Takahashi, Y. Tamada, and T. Yokochi. 1996. Apoptotic cell death in the response of D-galactosamine-sensitized mice to lipopolysaccharide as an experimental endotoxic shock model. Infection and Immunity 64 (3): 734–738.
Bahjat, F.R., V.R. Dharnidharka, K. Fukuzuka, L. Morel, J.M. Crawford, M.J. Clare-Salzler, and L.L. Moldawer. 2000. Reduced susceptibility of nonobese diabetic mice to TNF-alpha and D-galactosamine-mediated hepatocellular apoptosis and lethality. Journal of Immunology 165 (11): 6559–6567.
Nowak, M., G.C. Gaines, J. Rosenberg, R. Minter, F.R. Bahjat, J. Rectenwald, S.L. MacKay, C.K. Edwards, and L.L. Moldawer. 2000. LPS-induced liver injury in D-galactosamine-sensitized mice requires secreted TNF-alpha and the TNF-p55 receptor. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 278 (5): 1202–1209.
Takahashi, N., L. Vereecke, M.J. Bertrand, L. Duprez, S.B. Berger, T. Divert, A. Gonçalves, et al. 2014. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature 513 (7516): 95–99. doi:10.1038/nature13706.
Suda, J., L. Dara, L. Yang, M. Aghajan, Y. Song, N. Kaplowitz, and Z.X. Liu. 2016. Knockdown of RIPK1 markedly exacerbates murine immune-mediated liver injury through massive apoptosis of hepatocytes, independent of necroptosis and inhibition of NF-κB. Journal of Immunology 197 (8): 3120–3129. doi:10.4049/jimmunol.1600690.
Filliol, A., C. Piquet-Pellorce, C. Raguénès-Nicol, S. Dion, M. Farooq, C. Lucas-Clerc, P. Vandenabeele, M.J.M. Bertrand, J. Le Seyec, and M. Samson. 2017. RIPK1 protects hepatocytes from Kupffer cells-mediated TNF-induced apoptosis in mouse models of PAMP-induced hepatitis. Journal of Hepatology 66 (6): 1205–1213. doi:10.1016/j.jhep.2017.01.005.
Filliol, A., C. Piquet-Pellorce, J. Le Seyec, M. Farooq, V. Genet, C. Lucas-Clerc, J. Bertin, et al. 2016. RIPK1 protects from TNF-α-mediated liver damage during hepatitis. Cell death and disease 7 (11): e2462. doi:10.1038/cddis.2016.362.
Schneider, A.T., J. Gautheron, M. Feoktistova, C. Roderburg, S.H. Loosen, S. Roy, F. Benz, et al. 2017. RIPK1 suppresses a TRAF2-dependent pathway to liver cancer. Cancer Cell 31 (1): 94–109. doi:10.1016/j.ccell.2016.11.009.
Chen, G., J. Li, M. Ochani, B. Rendon-Mitchell, X. Qiang, S. Susarla, L. Ulloa, et al. 2004. Bacterial endotoxin stimulates macrophages to release HMGB1 partly through CD14- and TNF-dependent mechanisms. Journal of Leukocyte Biology 76 (5): 994–1001. doi:10.1189/jlb.0404242.
Gardella, S., C. Andrei, D. Ferrera, L.V. Lotti, M.R. Torrisi, M.E. Bianchi, and A. Rubartelli. 2002. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Reports 3 (10): 995–1001. doi:10.1093/embo-reports/kvf198.
Pavlosky, A., A. Lau, Y. Su, D. Lian, X. Huang, Z. Yin, A. Haig, A.M. Jevnikar, and Z.X. Zhang. 2014. RIPK3-mediated necroptosis regulates cardiac allograft rejection. American Journal of Transplantation 14 (8): 1778–1790. doi:10.1111/ajt.12779.
Qing, D.Y., D. Conegliano, M.G. Shashaty, J. Seo, J.P. Reilly, G.S. Worthen, D. Huh, N.J. Meyer, and N.S. Mangalmurti. 2014. Red blood cells induce necroptosis of lung endothelial cells and increase susceptibility to lung inflammation. American Journal of Respiratory and Critical Care Medicine 190 (11): 1243–1254. doi:10.1164/rccm.201406-1095OC.
Lefrancais, E., and C. Cayrol. 2012. Mechanisms of IL-33 processing and secretion: differences and similarities between IL-1 family members. European Cytokine Network 23 (4): 120–127. doi:10.1684/ecn.2012.0320.
Rickard, J.A., J.A. O’Donnell, J.M. Evans, N. Lalaoui, A.R. Poh, T. Rogers, J.E. Vince, et al. 2014. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 157 (5): 1175–1188. doi:10.1016/j.cell.2014.04.019.
Arshad, M.I., C. Piquet-Pellorce, and M. Samson. 2012. IL-33 and HMGB1 alarmins: sensors of cellular death and their involvement in liver pathology. Liver International 32 (8): 1200–1210. doi:10.1111/j.1478-3231.2012.02802.x.
Arshad, M.I., C. Piquet-Pellorce, A. Filliol, A. L’Helgoualc’h, C. Lucas-Clerc, S. Jouan-Lanhouet, M.T. Dimanche-Boitrel, and M. Samson. 2015. The chemical inhibitors of cellular death, PJ34 and necrostatin-1, down-regulate IL-33 expression in liver. Journal of Molecular Medicine 93 (8): 867–878. doi:10.1007/s00109-015-1270-6.
Liu, Z.Y., B. Wu, Y.S. Guo, Y.H. Zhou, Z.G. Fu, B.Q. Xu, J.H. Li, et al. 2015. Necrostatin-1 reduces intestinal inflammation and colitis-associated tumorigenesis in mice. American Journal of Cancer Research 5 (10): 3174–3185.
Humphries, F., S. Yang, B. Wang, and P.N. Moynagh. 2015. RIP kinases: key decision makers in cell death and innate immunity. Cell Death and Differentiation 22 (2): 225–236. doi:10.1038/cdd.2014.126.
Kim, S.J., and J. Li. 2013. Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell death and disease 4: e716. doi:10.1038/cddis.2013.238.
Wang, F., Z. Lu, M. Hawkes, H. Yang, K.C. Kain, and W.C. Liles. 2010. Fas (CD95) induces rapid, TLR4/IRAK4-dependent release of pro-inflammatory HMGB1 from macrophages. Journal of Inflammation 7: 30. doi:10.1186/1476-9255-7-30.
Duprez, L., N. Takahashi, F. Van Hauwermeiren, B. Vandendriessche, V. Goossens, T. Vanden Berghe, W. Declercq, C. Libert, A. Cauwels, and P. Vandenabeele. 2011. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35 (6): 908–918. doi:10.1016/j.immuni.2011.09.020.
Zhang, Q., M. Raoof, Y. Chen, Y. Sumi, T. Sursal, W. Junger, K. Brohi, K. Itagaki, and C.J. Hauser. 2010. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464 (7285): 104–107. doi:10.1038/nature08780.
Brenner, C., L. Galluzzi, O. Kepp, and G. Kroemer. 2013. Decoding cell death signals in liver inflammation. Journal of Hepatology 59 (3): 583–594. doi:10.1016/j.jhep.2013.03.033.
Chen, R., W. Hou, Q. Zhang, R. Kang, X.G. Fan, and D. Tang. 2013. Emerging role of high-mobility group box 1 (HMGB1) in liver diseases. Molecular Medicine 19: 357–366. doi:10.2119/molmed.2013.00099.
Wang, H., O. Bloom, M. Zhang, J.M. Vishnubhakat, M. Ombrellino, J. Che, A. Frazier, et al. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285 (5425): 248–251. doi:10.1126/science.285.5425.248.
Xu, J., Y. Jiang, J. Wang, X. Shi, Q. Liu, Z. Liu, Y. Li, et al. 2014. Macrophage endocytosis of high mobility group box 1 triggers pyroptosis. Cell Death and Differentiation 21 (8): 1229–1239. doi:10.1038/cdd.2014.40.
Huebener, P., J.P. Pradere, C. Hernandez, G.Y. Gwak, J.M. Caviglia, X. Mu, J.D. Loike, R.E. Jenkins, D.J. Antoine, and R.F. Schwabe. 2015. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. Journal of Clinical Investigation 125 (2): 539–550. doi:10.1172/JCI76887.
Kuhla, A., J. Norden, K. Abshagen, M.D. Menger, and B. Vollmar. 2013. RAGE blockade and hepatic microcirculation in experimental endotoxaemic liver failure. British Journal of Surgery 100 (9): 1229–1239. doi:10.1002/bjs.9188.
Matsumura, T., A. Ito, T. Takii, H. Hayashi, and K. Onozaki. 2000. Endotoxin and cytokine regulation of toll-like receptor (TLR) 2 and TLR4 gene expression in murine liver and hepatocytes. Journal of Interferon & Cytokine Research 20 (10): 915–921. doi:10.1089/10799900050163299.
Cho, H.I., J.M. Hong, J.W. Choi, H.S. Choi, J.H. Kwak, D.W. Lee, S.K. Lee, and S.M. Lee. 2015. β-Caryophyllene alleviates D-galactosamine and lipopolysaccharide-induced hepatic injury through suppression of the TLR4 and RAGE signaling pathways. European Journal of Pharmacology 764: 613–621. doi:10.1016/j.ejphar.2015.08.001.
Kitazawa, T., T. Tsujimoto, H. Kawaratani, and H. Fukui. 2010. Salvage effect of E5564, Toll-like receptor 4 antagonist on D-galactosamine and lipopolysaccharide-induced acute liver failure in rats. Journal of Gastroenterology and Hepatology 25 (5): 1009–1012. doi:10.1111/j.1440-1746.2009.06145.x.
Ben Ari, Z., O. Avlas, O. Pappo, V. Zilbermints, Y. Cheporko, L. Bachmetov, R. Zemel, et al. 2012. Reduced hepatic injury in Toll-like receptor 4-deficient mice following D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure. Cellular Physiology and Biochemistry 29 (1–2): 41–50. doi:10.1159/000337585.
Ott, C., K. Jacobs, E. Haucke, A. Navarrete Santos, T. Grune, and A. Simm. 2014. Role of advanced glycation end products in cellular signaling. Redox Biology 2: 411–429. doi:10.1016/j.redox.2013.12.016.
Nadatani, Y., T. Watanabe, T. Tanigawa, F. Ohkawa, S. Takeda, A. Higashimori, M. Sogawa, et al. 2013. High-mobility group box 1 inhibits gastric ulcer healing through Toll-like receptor 4 and receptor for advanced glycation end products. PloS One 8 (11): e80130. doi:10.1371/journal.pone.0080130.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2013R1A1A3008145).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Kim, SJ., Lee, SM. Necrostatin-1 Protects Against d-Galactosamine and Lipopolysaccharide-Induced Hepatic Injury by Preventing TLR4 and RAGE Signaling. Inflammation 40, 1912–1923 (2017). https://doi.org/10.1007/s10753-017-0632-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0632-3