ABSTRACT
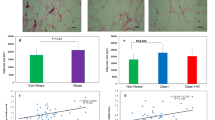
S100A9 (calgranulin B) has inflammatory and oxidative stress properties and was found to be associated with atherosclerosis and obesity. One of the proteins that can regulate S100A9 transcription is p53, which is involved in cell cycle, apoptosis and adipogenesis. Thus, it triggers adipocyte enlargement and finally obesity. Because epicardial adipose tissue (EAT) volume and thickness is related to coronary artery disease (CAD), we studied the gene expression of this pathway in patients with cardiovascular disease and its association with obesity. Adipocytes and stromal cells from EAT and subcutaneous adipose tissue (SAT) from 48 patients who underwent coronary artery bypass graft and/or valve replacement were obtained after collagenase digestion and differential centrifugation. The expression levels of the involved genes on adipogenesis and cell cycle like fatty acid-binding protein (FABP) 4, retinol-binding protein (RBP)4, p53 and S100A9 were determined by real-time polymerase chain reaction (PCR). Adipocyte diameter was measured by optical microscopy. We found that epicardial adipocytes expressed significantly lower levels of adipogenic genes (FABP4 and RBP4) and cell cycle-related genes (S100A9 and p53) than subcutaneous adipocytes. However, in obese patients, upregulation of adipogenic and cell cycle-related genes in subcutaneous and epicardial adipocytes, respectively, was observed. The enlargement of adipocyte size was related to FABP4, S100A9 and p53 expression levels in stromal cells. But only the p53 association was maintained in epicardial stromal cells from obese patients (p = 0.003). The expression of p53, but not S100A9, in epicardial stromal cells is related to adipocyte enlargement in obese patients with cardiovascular disease. These findings suggest new mechanisms for understanding the relationship between epicardial fat thickness, obesity and cardiovascular disease.





Similar content being viewed by others
References
Nakanishi, R., R. Rajani, V.Y. Cheng, H. Gransar, R. Nakazato, H. Shmilovich, et al. 2011. Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: a serial study using non-contrast cardiac CT. Atherosclerosis 218: 363–368.
Ahn, S.G., H.S. Lim, D.Y. Joe, S.J. Kang, B.J. Choi, S.Y. Choi, et al. 2008. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart 94: e7.
Yerramasu, A., D. Dey, S. Venuraju, D.V. Anand, S. Atwal, R. Corder, et al. 2012. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis 220: 223–230.
Kanda, H., S. Tateya, Y. Tamori, K. Kotani, K. Hiasa, R. Kitazawa, et al. 2006. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of Clinical Investigation 116: 1494–1505.
Kern, P.A., S. Ranganathan, C. Li, L. Wood, and G. Ranganathan. 2001. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. American Journal of Physiology - Endocrinology and Metabolism 280: E745–E751.
Fantuzzi, G. 2005. Adipose tissue, adipokines, and inflammation. The Journal of Allergy and Clinical Immunology 115: 911–919. quiz 920.
Hajer, G.R., T.W. van Haeften, and F.L. Visseren. 2008. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European Heart Journal 29: 2959–2971.
Skurk, T., C. Alberti-Huber, C. Herder, and H. Hauner. 2007. Relationship between adipocyte size and adipokine expression and secretion. The Journal of Clinical Endocrinology and Metabolism 92: 1023–1033.
Barazzoni, R., G. Biolo, M. Zanetti, A. Bernardi, and G. Guarnieri. 2006. Inflammation and adipose tissue in uremia. Journal of Renal Nutrition 16: 204–207.
Findeisen, H.M., K.J. Pearson, F. Gizard, Y. Zhao, H. Qing, K.L. Jones, et al. 2011. Oxidative stress accumulates in adipose tissue during aging and inhibits adipogenesis. PLoS One 6: e18532.
Eiras, S., E. Teijeira-Fernandez, L.G. Shamagian, A.L. Fernandez, A. Vazquez-Boquete, and J.R. Gonzalez-Juanatey. 2008. Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue. Cytokine 43: 174–180.
Teijeira-Fernandez, E., S. Eiras, L. Grigorian-Shamagian, A. Fernandez, B. Adrio, and J.R. Gonzalez-Juanatey. 2008. Epicardial adipose tissue expression of adiponectin is lower in patients with hypertension. Journal of Human Hypertension 22: 856–863.
Catalan, V., J. Gomez-Ambrosi, A. Rodriguez, B. Ramirez, F. Rotellar, V. Valenti, et al. 2011. Increased levels of calprotectin in obesity are related to macrophage content. Impact on inflammation and effect of weight loss. Molecular Medicine 17: 1157–1167.
Averill, M.M., S. Barnhart, L. Becker, X. Li, J.W. Heinecke, R.C. Leboeuf, et al. 2011. S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation. Circulation 123: 1216–1226.
Zhao, F., B. Hoechst, A. Duffy, J. Gamrekelashvili, S. Fioravanti, M.P. Manns, et al. 2012. S100A9 a new marker for monocytic human myeloid derived suppressor cells. Immunology 136: 176–183.
Li, C., H. Chen, F. Ding, Y. Zhang, A. Luo, M. Wang, et al. 2009. A novel p53 target gene, S100A9, induces p53-dependent cellular apoptosis and mediates the p53 apoptosis pathway. The Biochemical Journal 422: 363–372.
Molchadsky, A., I. Shats, N. Goldfinger, M. Pevsner-Fischer, M. Olson, A. Rinon, et al. 2008. p53 plays a role in mesenchymal differentiation programs, in a cell fate dependent manner. PLoS One 3: e3707.
Fain, J.N., A.K. Madan, M.L. Hiler, P. Cheema, and S.W. Bahouth. 2004. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 145: 2273–2282.
Bruun, J.M., A.S. Lihn, S.B. Pedersen, and B. Richelsen. 2005. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. The Journal of Clinical Endocrinology and Metabolism 90: 2282–2289.
Hotamisligil, G.S. 2006. Inflammation and metabolic disorders. Nature 444: 860–867.
Tchoukalova, Y.D., C. Koutsari, M.V. Karpyak, S.B. Votruba, E. Wendland, and M.D. Jensen. 2008. Subcutaneous adipocyte size and body fat distribution. The American Journal of Clinical Nutrition 87: 56–63.
Wellen, K.E., and G.S. Hotamisligil. 2003. Obesity-induced inflammatory changes in adipose tissue. The Journal of Clinical Investigation 112: 1785–1788.
Dandona, P., A. Aljada, A. Chaudhuri, P. Mohanty, and R. Garg. 2005. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 111: 1448–1454.
Chatterjee, T.K., L.L. Stoll, G.M. Denning, A. Harrelson, A.L. Blomkalns, G. Idelman, et al. 2009. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circulation Research 104: 541–549.
Eiras, S., E. Teijeira-Fernandez, A. Salgado-Somoza, E. Couso, T. Garcia-Caballero, J. Sierra, et al. 2010. Relationship between epicardial adipose tissue adipocyte size and MCP-1 expression. Cytokine 51: 207–212.
Salgado-Somoza, A., E. Teijeira-Fernandez, A.L. Fernandez, J.R. Gonzalez-Juanatey, and S. Eiras. 2010. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. American Journal of Physiology. Heart and Circulatory Physiology 299: H202–H209.
Mazurek, T., L. Zhang, A. Zalewski, J.D. Mannion, J.T. Diehl, H. Arafat, et al. 2003. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108: 2460–2466.
Hirata, Y., H. Kurobe, M. Akaike, F. Chikugo, T. Hori, Y. Bando, et al. 2011. Enhanced inflammation in epicardial fat in patients with coronary artery disease. International Heart Journal 52: 139–142.
McCormick, M.M., F. Rahimi, Y.V. Bobryshev, K. Gaus, H. Zreiqat, H. Cai, et al. 2005. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. The Journal of Biological Chemistry 280: 41521–41529.
Rodino-Janeiro, B.K., A. Salgado-Somoza, E. Teijeira-Fernandez, J.R. Gonzalez-Juanatey, E. Alvarez, and S. Eiras. 2011. Receptor for advanced glycation end-products expression in subcutaneous adipose tissue is related to coronary artery disease. European Journal of Endocrinology 164: 529–537.
Hofmann, M.A., S. Drury, C. Fu, W. Qu, A. Taguchi, Y. Lu, et al. 1999. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97: 889–901.
Bazuine, M., K.G. Stenkula, M. Cam, M. Arroyo, and S.W. Cushman. 2009. Guardian of corpulence: a hypothesis on p53 signaling in the fat cell. Clin Lipidol 4: 231–243.
Acknowledgments
The present study was supported by Complejo Hospitalario Universitario de Santiago de Compostela (Santiago de Compostela, Spain). Dr. Eiras is a researcher within the Isidro Parga Pondal Program (Xunta de Galicia, Spain) and European Social Fund (ESF). This study was supported by Xunta de Galicia project (PGIDIT10-PXIB918076PR).
Author information
Authors and Affiliations
Corresponding author
ELECTRONIC SUPPLEMENTARY MATERIAL
Below is the link to the electronic supplementary material.
ESM 1
(JPEG 52 kb)
Rights and permissions
About this article
Cite this article
Agra, R.M., Fernández-Trasancos, Á., Sierra, J. et al. Differential Association of S100A9, an Inflammatory Marker, and p53, a Cell Cycle Marker, Expression with Epicardial Adipocyte Size in Patients with Cardiovascular Disease. Inflammation 37, 1504–1512 (2014). https://doi.org/10.1007/s10753-014-9876-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-9876-3