Abstract
Influenza A virus pandemics and emerging antiviral resistance highlight the urgent need for novel generic pharmacological strategies that reduce both viral replication and inflammation of the lung. We have previously investigated the therapeutic efficacy of recombinant human catalase (rhCAT) against viral pneumonia in mice, but the protection mechanisms involved were not explored. In the present study, we have performed a more in-depth analysis covering survival, lung inflammation, immune cell responses, production of cytokines, and inflammation signaling pathways in mice. Male imprinting control region mice were infected intranasally with high pathogenicity (H1N1) influenza A virus followed by treatment with recombinant human catalase. The administration of rhCAT resulted in a significant reduction in inflammatory cell infiltration (e.g., macrophages and neutrophils), inflammatory cytokine levels (e.g., IL-2, IL-6, TNF-α, IFN-γ), the level of the intercellular adhesion molecule 1 chemokine and the mRNA levels of toll-like receptors TLR-4, TLR-7, and NF-κB, as well as partially maintaining the activity of the antioxidant enzymes system. These findings indicated that rhCAT might play a key protective role in viral pneumonia of mice via suppression of inflammatory immune responses.
Similar content being viewed by others
INTRODUCTION
Reactive oxygen species (ROS) have been implicated in the pathogenesis of many diseases such as human immunodeficiency virus infection [1]. Although ROS function is a beneficial component of the immune response, unregulated and excessive ROS are toxic, causing irreparable damage to the cellular membrane, proteins, and nucleic acids. Under normal physiological conditions, antioxidant enzymes help to maintain the balance of ROS. This functional interaction has led to an interest in generating antioxidants as therapeutic agents for ROS-mediated injury and diseases.
According to global estimates generated by the World Health Organization, 450 million cases of pneumonia are diagnosed every year. Approximately 4 million of those cases end in death, accounting for 7 % of total mortality worldwide. The recent emergence of severe acute respiratory syndrome (SARS)-associated coronavirus, avian influenza A (H5N1) virus, the 2009 pandemic influenza A (H1N1) virus and novel avian influenza A H7N9 virus [2–4] has served to highlight respiratory viruses as important causative agents of severe pneumonia. Nonetheless, the current battery of antivirals available in clinical practice for the treatment of pneumonia is limited [5]. The continued pandemic threat of these circulating viruses makes identification and development of novel therapeutic strategies, especially for the treatment of influenza A, an urgent matter
Recent evidence suggests that much of the acute lung injury caused by H1N1 and H5N1 can be attributed to excessive ROS production (i.e., oxidative stress) initiated by an overactive innate immune response [6–8]. ROS, including the superoxide anion, hydrogen peroxide (H2O2) and the hydroxyl radical (OH·), are indiscriminately toxic to cells when produced in excess and are capable of regulating proinflammatory cytokine production. A major cellular source of ROS during infection is infiltrating inflammatory cells [9]. Identification of the enzymatic sources of ROS may pave the way for therapies that combat the oxidative stress-dependent lung injury caused by the influenza A virus.
Catalase (CAT) is one of the key antioxidant enzymes of the mammalian system. CAT works by catalyzing the decomposition of H2O2 into molecular oxygen and water, and plays an important role in the development of tolerance to oxidative stress. This process is protective against several pathological conditions. The potential of CAT as a therapeutic agent has been explored for many different diseases and in many different forms, including gene therapy and as recombinant CAT, for well over a decade [10–14].
In our previous study, we found that inhalation of native and PEGylated recombinant human catalase (rhCAT) elicits a protective effect in mice with influenza-associated pneumonia [15, 16]. However, it remains to be determined if rhCAT influences (1) the innate immune responses that control and clear the influenza virus, (2) inflammatory cytokines, and/or (3) possible inflammation signaling pathways. The present study showed that rhCAT is a novel and potential alternative for the control of influenza infection and may be a way of controlling future pandemics by modulating the host instead of the virus.
MATERIALS AND METHODS
Experimental infection of mice with H1N1 influenza virus
Imprinting control region (ICR) male mice, at 16–18 g, were purchased from the Shanghai SLACCAS Laboratory Animal Co., Ltd. (Shanghai, China). Mice were housed under specific pathogen-free conditions and given free access to sterile water and standard mouse chow. All experimental protocols were approved by the Animal Experiment Committee of Fudan University (Shanghai, China).
The influenza virus A/FM/1/47 (H1N1) used in this study is a highly virulent, mouse-adapted virus isolated from patients at Fort Monmouth, NJ, USA during an outbreak in 1947 that can cause severe pneumonia and high mortality in mice. The virus was supplied by the Shanghai Center for Disease Control & Prevention (Shanghai, China) and stored in aliquots at −70 °C. For each experiment, an aliquot was thawed to ensure use of a fresh preparation [17].
The mice were randomly divided into groups: (1) virus-infected, rhCAT-treated (100, 50, 25 kU/kg); (2) virus-infected, saline-treated controls (virus controls); and (3) uninfected, saline-treated controls (normal controls). Mice under isoflurane anesthesia were infected intranasally (i.n.) with 8 TCID50 [18] of influenza virus A/FM/1/47 (H1N1). Two hours post-infection, mice were intranasally inoculated with either 30 μL of 0.9 % NaCl saline or rhCAT (dissolved in saline) twice daily. The rhCAT was obtained according to published methods [19].
For the survival study (n = 10 mice per group), treatments were administered as described above for 7 days. Then, mice were continuously monitored for survival over the next 7 days. Survival analysis curves were generated by plotting the number of survivors divided by the total number of mice in a group (expressed as percentage of mice that survived). Mice were also monitored for body weight loss for 4 days post-infection.
Histopathology and lung/body weight
For histopathology, further experimental infections as described above were performed. On day 4 post-infection, the remaining mice (n = 6, per group) were euthanized and weighed. Lung tissues were harvested and weighed and the corresponding lung/body index was calculated. The left lobes of the lung were suspended in PBS-buffered formalin. They were then preserved in paraffin blocks using standard procedures. Then, 10-μm tissue sections were cut, placed on glass slides, and stained with hematoxylin and eosin using standard techniques. Microscopic analysis was carried out by three separate pathologists who were blinded to the various experimental treatments.
Oxidative injury and major antioxidant status
For antioxidant status analysis, mouse lung homogenates from 100 mg of the lobes of the lungs (days 2 and 4) were freshly prepared and diluted to 1 ml in PBS solution. Total protein content, malondialdehyde (MDA) concentration, total antioxidant status (TAS), superoxide dismutase (SOD) concentration, and CAT concentrations were determined using commercially available assay kits (Kit # A45-3, A015, A003-2, A001-1, and A007, respectively; Jiancheng Bioengineering Institute, China).
Cellular Analysis by Flow Cytometry
For cellular analysis, inflammatory cytokines measurement and signaling pathway analysis, further experimental infections as described above were performed, but the mice only received 100 kU/kg dosage of rhCAT.
On days 2 and 4 post-infection, the mice (n = 4, per group) were euthanized. Lungs were harvested from PBS-perfused mice and diced using surgical scissors. Diced tissue was suspended in 4 mL of CDTI buffer [0.5 mg/mL collagenase from Clostridium histolyticum type IV (Sigma), 50 U/mL Dnase I (Sigma), 1 mg/mL trypsin inhibitor type Ii-s (Sigma) in DMEM] for 1 h at 37 °C. The suspension was then passed through a 100-μm filter, and unwanted red blood cells were lysed using red blood cell lysis buffer [0.02 Tris–HCl (pH 7.4), 0.14 NH4Cl]. Inflammatory cells were purified by centrifugation in 35 % PBS-buffered Percoll (GE Healthcare Life Sciences) at 500 × g for 15 min. Cell pellets were resuspended in staining buffer (RPMI-1640 medium), and Fc receptors were blocked using 25-μg/mL anti-mouse CD16/32 (BD Biosciences). Cells were stained with fluorescently labeled antibodies against the following mouse proteins: CD11b+, F480−, Ly6G+ (Neutrophils), CD11b+, F480+, and Ly6G− (Macrophage/monocytes).
Inflammatory cytokine measurement
Tissue concentrations of the murine cytokines TNF-α, IFN-γ, IL-2, IL-6 and the Intercellular Adhesion Molecule-1 (ICAM-1) were determined using commercial ELISA kits (BD Biosciences) following the manufacturer's instructions.
Measurement of mRNA levels of the TLR-4, TLR-7 and NF-κB p65 genes
The isolation of total RNA and cDNA synthesis were performed using Trizol reagent (Invitrogen) and PrimeScript® RT reagent Kit (DRR047A, Takara) according to the manufacturer's recommendations. The RT primers for TLR-4, TLR-7, NF-κB and GAPDH were as follows: TLR-4 Forward: 5′-GCACTGTTCTTCTCCTGCC-3′, TLR-4 Reverse: 5′-GTTTCCTGTCAGTATCAAG-3′; TLR-7 Forward: 5′-GGTGGCAAAATTGGAAGATCC-3′, TLR7 Reverse: 5′-AGCTGTATGCTCTGGGAAAGGTT-3′; NF-κB p65 Forward: 5′-ATGTGCATCGGCAAGTGG-3′, NF-κB p65 Reverse: 5′-CAGAAGTTGAGTTTCGGGTAG-3′; GAPDH Forward: 5-ACCCACTCCTCCACCTTTGA-3, GAPDH Reverse: 5-CTGTTGCTGTAGCCAAATTCGT-3. Using cDNAs as the template, quantitative real-time PCR was carried out using the SYBR Green PCR Master Mix (Applied Biosystem) in a StepOne Plus Real-Time PCR Detection System (Applied Biosystems), according to the manufacturer's instructions and with the following thermocycling parameters: 94 °C for 5 min; followed by 94 °C for 5 s, 60 °C for 30 s for 40 cycles with a final melting curve analysis of 60–95 °C. The mRNA expression levels were normalized to the corresponding expression level of the GAPDH housekeeping gene.
Statistical analyses
All statistical analyses were performed using GraphPad Prism for Windows (Version 6.0). The Gehan-Breslow-Wilcoxon test was used to analyze the survival of mice while the one-way ANOVA was used to analyze other experimental data. In all cases, probability levels less than 0.05 (p < 0.05) were taken to indicate statistical significance.
RESULTS
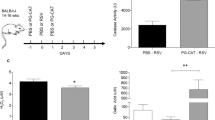
rhCAT administration significantly improved survival by limiting pulmonary injury
Mice were treated with either saline or rhCAT intranasally 2 h after intranasal infection with the mouse-adapted human influenza virus A/FM/1/47 (H1N1). rhCAT administration significantly and in a dose-dependent manner lengthened the survival time of mice, compared to the virus control mice (Fig. 1a). For recipients of saline alone, no mice in the virus control group survived beyond 6 day post-infection, whereas 35 % of mice that received rhCAT (100 kU) survived until 14 day post-infection (p < 0.01). In mice treated with rhCAT, body weight loss was also reduced in a dose-dependent manner when measured on day 4 post-infection (p < 0.05, Fig. 1b).
rhCAT administration significantly protects against pathogenic H1N1 influenza virus infection and inhibits the immunopathological effects associated with infection. Saline or 100 kU/kg rhCAT was administered i.n. to mice 2 h after i.n. infection with 8 TCID50 influenza virus A/FM/1/47 (H1N1). a Mice were monitored for survival. For recipients of saline alone, no mice survived beyond 14 days post-infection, whereas 35 % of mice that received rhCAT survived until 14 day post-infection (p < 0.05). Data were derived from three separate experiments with a total of 30 mice per group. Data below individual survival curves represent the number of survivors/total number of mice (i.e., percentage of mice that survived). Survival curves show data until day 14 post-infection because further mortality was not observed at later time points. b Body weight of infected mice was monitored from day 0 to day 4 post-infection, which presents the tolerance to virus infection under different treatments. c–d Lung/body weight and total protein content were evaluated on day 4 post-infection. Mice were weighed (grams) and euthanized. Whole lungs were harvested, weighed (milligrams) and the corresponding lung/body index was calculated. Lung tissue (100 mg) was homogenized and diluted to 1 ml in PBS for the total protein assay. Lung/body weight and total protein content indicate the severity of the lung tissue exudate. e–i Influenza virus induced severe interstitial pneumonia (the black arrows indicate the mononuclear inflammatory cells infiltrating the wall of the alveoli) and bronchiolitis (the red arrows indicate inflammation of the bronchioles with mononuclear inflammatory cell infiltration). Histopathological analysis on day 4 post-infection revealed diminished inflammation characterized by reduced mononuclear cell infiltration, alveolitis, bronchiolitis, vascular hemorrhaging and edema in rhCAT-treated mice (100, 50, 25 kU/kg, g–i) compared with virus controls (f).
Lung/body index (Fig. 1c) and total protein content in lung tissue (Fig. 1d) demonstrated that H1N1 virus infection caused lung tissue swelling and the production of significant amounts of protein exudate in the control mice, while rhCAT administration (50 and 100 kU) significantly alleviated these effects. Histopathological analysis of lungs from the infected mice treated with rhCAT revealed markedly reduced tissue injury, mononuclear cell accumulation, hemorrhage, and pulmonary edema, compared with virus control mice on day 4 post-infection (Fig. 1e–i).
rhCAT administration partly restored the levels of antioxidant enzymes and alleviated tissue peroxidation injury
Viral replication can induce excessive oxidative responses thereby exhausting the antioxidant pool. For this reason, the levels of key antioxidant enzymes [e.g., CAT and superoxide dismutase (SOD)], total antioxidant status (TAS), and MDA (the product of lipid peroxidation representing ROS-mediated tissue peroxidation injury) were evaluated in lung tissue on days 2 and 4 post-infection. Influenza infection induced a significant increase of MDA and a decrease in CAT and SOD activity in virus control mice while the administration of rhCAT resulted in a dose-dependent reduction of MDA and a higher activity of CAT and SOD (Fig. 2; p ≤ 0.05). rhCAT treatment slightly influenced the level of TAS but revealed no significant difference between virus control and rhCAT-treated mice (Fig. 2b). The antioxidant system is comprised of non-enzymatic antioxidant molecules (e.g., vitamins A, C, and E, glutathione, etc.) and antioxidant enzymes (e.g., CAT, SOD and various peroxidases) and is therefore reflected in TAS measurements. This therefore indicates that rhCAT may partly restore the antioxidant enzyme system while at the same time not significantly influencing TAS levels. Thus. rhCAT administration reduced ROS-mediated injury, which might be relevant in alleviating the effects of viral pneumonia, thereby reducing mortality.
rhCAT partially restored the key antioxidant enzymes content and diminished the tissue peroxidation injury. On days 2 and 4 post-infection, mice were euthanized and the lungs were harvested. Lung tissue (100 mg) was homogenized and assayed for the following: a Malondialdehyde (MDA) is a marker for oxidative stress. The content of MDA represents the level of ROS production in virus-infected mice. b Total antioxidant status. c–d Key antioxidant enzymes (CAT and SOD). Data are presented as mean ± SD. # p ≤ 0.05 compared with saline recipients.
In conclusion, rhCAT can protect against influenza virus infection in a dose-dependent manner. Thus, 100 kU/kg rhCAT was used to explore the possible protection mechanisms of rhCAT in influenza virus infection.
rhCAT inhibited the burst of inflammatory cells and cytokines by downregulating key genes in the TLR-NF-κB signaling pathway
Robust innate proinflammatory cytokine expression can cause direct tissue insult and recruit potentially tissue-destructive inflammatory cells. Lung tissue insult and a high inflammatory cell infiltration were observed during the histopathological analysis. Analysis of neutrophils and macrophages/monocytes also revealed a significant reduction in the accumulation of neutrophils (CD11b+, F480−, Ly6G+) and macrophage/monocytes (CD11b+, F480+, Ly6G−) in the lung 4 days post-infection (Fig. 3a).
rhCAT reduced inflammatory cells infiltration and dampened the production of proinflammatory cytokines by downregulating key genes in the NFκB signaling pathway. On days 2 and 4 post-infection, mice were euthanized and the lungs were harvested and assayed for the following: a inflammatory cells by flow cell cytometry (e.g., neutrophils and macrophage/monocytes). b–c Inflammatory cytokines (TNF-α, IFN-γ, IL-2, IL-6, ICAM-1) were analyzed by ELISA. d Key genes (TLR4, TLR7, NF-κB p65, responding to H1N1-infection) in NFκB signaling pathway were analyzed by qPCR. Data are presented as mean ± SD. # p ≤ 0.05 compared with virus controls (saline recipients).
Analysis of cytokines in rhCAT-treated mice revealed significant reductions in IFN-γ, IL-6, TNF-α, IL-2, ICAM-1 on the fourth day post-infection compared with the control mice (Fig. 3b, c). Immune cell infiltration and activation can be affected by a reduction in cytokine production in rhCAT-treated mice.
We also analyzed the proinflammatory cytokine (TLR-NF-κB) signaling pathway in H1N1 virus-infected mice by qPCR. The results showed that H1N1 infection significantly upregulated the specific influenza recognition receptor TLR7 and NF-κB at the transcriptional level in the control mice while treatment with rhCAT inhibited their expression. Interestingly, the mRNA level of TLR4, the typical lipopolysaccharide (LPS) recognition receptor, was dramatically increased in virus-infected mice, which was also inhibited by rhCAT (Fig. 3d). This indicates that rhCAT downregulates the TLR-NF-κB signaling pathway to suppress the inflammation responses induced by H1N1 infection.
DISCUSSION
In lethal influenza infections, severe viral pneumonia results in high morbidity and mortality, likely due to excessive production of ROS and aberrant cytokines [6–9]. In this study, H1N1 infection induced significant production of MDA and caused an obvious reduction of key antioxidant enzymes (CAT and SOD), which indirectly resulted in excessive ROS production in virus-infected mice. The administration of rhCAT significantly inhibited MDA production and increased the CAT and SOD levels (Fig. 3), suggesting that rhCAT administration partially diminished the excessive ROS production and protected the lungs from damage.
Aberrant and excessive cytokine production correlates with morbidity and mortality in macaques [20] and humans [21, 22] infected with highly virulent influenza viruses. Mouse models have demonstrated that many cytokines are essential for the control of virus replication but also exacerbate morbidity and tissue injury [23]. IFN-γ activates inflammatory cells and stimulates expression of multiple cytokines and chemokines [24–26]. IL-6 expression is directly linked to host morbidity [27, 28], and TNF-α secretion enhances pulmonary injury. Administration of rhCAT resulted in a significant reduction of cytokines (IFN-γ, IL-2, IL-6, TNF-α, and ICAM-1) in H1N1 influenza virus-infected mice (Fig. 3b–c), possibly due to the downregulation of TLR4, TLR7, and NF-kB at the transcriptional levels in the TLR-NF-κB signaling pathway (Fig. 3d). The mechanism of action of rhCAT was the blocking of cytokines/chemokines, as well as the blocking of infiltration and activation of inflammatory cells over the course of influenza virus infection, which resulted in diminished immune-mediated tissue injury and increased survival of the mice.
As a second messenger molecule, ROS also plays a key role in inflammation reactions induced by viruses [29, 30]. In the present work, the inflammatory cytokines (TNF-α, IFN-γ, IL-2, IL-6), chemokine ICAM-1 and transcriptional levels of key genes (TLR-4, TLR-7, NF-κB) in the TLR-NF-κB signaling pathway were significantly upregulated in the lung tissue of H1N1-infected mice. We think that the robust ROS reaction induced by H1N1 infection may have activated the TLR-NF-κB signaling pathway and stimulated the secretion of associated cytokines, resulting in severe pneumonia accompanied by a robust infiltration of inflammation-related cells in the control mice. This assumption echoes the latest finding that oxidized phospholipid potently stimulates TLR4-dependent inflammation [31]. The administration of rhCAT markedly suppressed inflammatory responses to H1N1-infection, which was probably ascribed to a decrease in ROS levels, but more direct experiments are needed in future studies.
There are still many aspects of this study that need to be investigated further, such as the possible influence of rhCAT on the murine immune system, innate antioxidant enzyme transcriptional levels, or other cellular responses (e.g., autophagy and apoptosis).
CONCLUSIONS
In summary, the administration of rhCAT provides an alternative and effective mechanism compared to antiviral drugs, and we envisage that use of rhCAT, in combination with antiviral strategies, may be an effective tool against morbidity and mortality induced by seasonal and pandemic strains of influenza A virus.
Abbreviations
- rhCAT:
-
Recombinant human catalase
- ICR:
-
Imprinting control region
- ROS:
-
Reactive oxygen species
- MDA:
-
Malondialdehyde
- TAS:
-
Total antioxidant status
- SOD:
-
Superoxide dismutase
- ICAM-1:
-
Intercellular adhesion molecule-1
- i.n.:
-
Intranasal
References
Deng, W., L. Baki, J. Yin, H. Zhou, and C.M. Baumgarten. 2000. HIV protease inhibitors elicit volume-sensitive Cl− current in cardiac myocytes via mitochondrial ROS. Journal of Molecular and Cellular Cardiology 49: 746–752.
Liu, D., W. Shi, Y. Shi, D. Wang, H. Xiao, W. Li, Y. Bi, Y. Wu, X. Li, J. Yan, W. Liu, G. Zhao, W. Yang, Y. Wang, J. Ma, Y. Shu, F. Lei, and G.F. Gao. 2013. Origin and diversity of novel avian influenza A H7N9 viruses causing human infection: phylogenetic, structural, and coalescent analyses. Lancet 381: 1926–1932.
Dai C, Jiang M: Understanding H7N9 avian flu. BMJ 2013, http:// dx.doi:10.1136/bmj.f2755.
Derek, G. 2009. The 2009 H1N1 influenza outbreak in its historical context. Journal of Clinical Virology 45: 174–178.
Ruuskanen, O., E. Lahti, L.C. Jennings, and D.R. Murdoch. 2011. Viral pneumonia. Lancet 9773: 1264–1275.
Imai, Y., K. Kuba, G.G. Neely, R. Yaghubian-Malhami, T. Perkmann, G. Van Loo, M. Ermolaeva, R. Veldhuizen, Y.H. Leung, H. Wang, H. Liu, Y. Sun, M. Pasparakis, M. Kopf, C. Mech, S. Bavari, J.S. Peiris, A.S. Slutsky, S. Akira, M. Hultqvist, R. Holmdahl, J. Nicholls, C. Jiang, C.J. Binder, and J.M. Penninger. 2008. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133: 235–249.
Oda, T., T. Akaike, T. Hamamoto, F. Suzuki, T. Hirano, and H. Maeda. 1989. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymerconjugated SOD. Science 244: 974–976.
Vlahos, R., J. Stambas, and S. Selemidis. 2012. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends in Pharmacological Sciences 33: 3–8.
Selemidis, S., C.G. Sobey, K. Wingler, H.H. Schmidt, and G.R. Drummond. 2008. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacology & Therapeutics 120: 254–291.
Ogbeyalu, E.O., E.J. George, Y.F. Zhao, L.C. Zhou, H. Yang, and Z.M. Guo. 2009. Overexpression of catalase delays G0/G1- to S phase transition during cell cycle progression in mouse aortic endothelial cells. Free Radical Biology & Medicine 46: 1658–1667.
Hanawa, T., S. Asayama, T. Watanabe, S. Owada, and H. Kawakami. 2009. Protective effects of the complex between manganese porphyrins and catalase-poly (ethylene glycol) conjugates against hepatic ischemia/reperfusion injury in vivo. Journal of Controlled Release 135: 60–64.
Nishikawa, M., M. Hashida, and Y. Takakura. 2009. Catalase delivery for inhibiting ROS-mediated tissue injury and tumor metastasis. Advanced Drug Delivery Reviews 61: 319–326.
Brown-Borg, H.M., and S.G. Rakoczy. 2000. Catalase expression in delayed and premature aging mouse models. Experimental Gerontology 35: 199–212.
Guy, J., X. Qi, and W.W. Hauswirth. 1998. Adeno-associated viral-mediated catalase expression suppresses optic neuritis in experimental allergic encephalomyelitis. Proceedings of the National Academy of Science 95: 13847–13852.
Shi, X.L., Z.H. Shi, H. Huang, H.G. Zhu, P. Zhou, and D.W. Ju. 2010. Therapeutic effect of recombinant human catalase on H1N1 influenza-induced pneumonia in mice. Inflammation 33: 166–172.
Shi X, Shi Z, Huang H, Zhu H, Zhu H, Ju D, Zhou P: PEGylated human catalase elicits potent therapeutic effects on H1N1 influenza-induced pneumonia in mice. Appl Microbiol Biotechnol 2013, http://dx.doi:10.1007/s00253-013-4775-3.
Michelle, D.T., R.J. Emma, G.B. Andrew, and C.R. Patrick. 2011. Glycosylation of the hemagglutinin modulates the sensitivity of H3N2 influenza viruses to innate proteins in airway secretions and virulence in mice. Virology 413: 84–92.
Liu, K., Z. Yao, L. Zhang, J. Li, L. Xing, and X. Wang. 2012. MDCK cell-cultured influenza virus vaccine protects mice from lethal challenge with different influenza viruses. Applied Microbiology and Biotechnology 94: 1173–1179.
Shi, X.L., M.Q. Feng, J. Shi, Z.H. Shi, J. Zhong, and P. Zhou. 2007. High-level expression and purification of recombinant human catalase in Pichia pastoris. Protein Expression and Purification 54: 24–29.
Kobasa, D., S.M. Jones, K. Shinya, J.C. Kash, J. Copps, H. Ebihara, Y. Hatta, J.H. Kim, P. Halfmann, M. Hatta, F. Feldmann, J.B. Alimonti, L. Fernando, Y. Li, M.G. Katze, H. Feldmann, and Y. Kawaoka. 2007. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445: 319–323.
Cillóniz, C., K. Shinya, X. Peng, M.J. Korth, S.C. Proll, L.D. Aicher, V.S. Carter, J.H. Chang, D. Kobasa, F. Feldmann, J.E. Strong, H. Feldmann, Y. Kawaoka, and M.G. Katze. 2009. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS Pathogens 5: e1000604.
de Jong, M.D., C.P. Simmons, T.T. Thanh, V.M. Hien, G.J. Smith, T.N. Chau, D.M. Hoang, N.V. Chau, T.H. Khanh, V.C. Dong, P.T. Qui, B.V. Cam, Q. Ha do, Y. Guan, J.S. Peiris, N.T. Chinh, T.T. Hien, and J. Farrar. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nature Medicine 12: 1203–1207.
Bermejo-Martin, J.F., R. Ortiz de Lejarazu, T. Pumarola, J. Rello, R. Almansa, P. Ramírez, I. Martin-Loeches, D. Varillas, M.C. Gallegos, C. Serón, D. Micheloud, J.M. Gomez, A. Tenorio-Abreu, M.J. Ramos, M.L. Molina, S. Huidobro, E. Sanchez, M. Gordón, V. Fernández, A. Del Castillo, M.A. Marcos, B. Villanueva, C.J. López, M. Rodríguez-Domínguez, J.C. Galan, R. Cantón, A. Lietor, S. Rojo, J.M. Eiros, C. Hinojosa, I. Gonzalez, N. Torner, D. Banner, A. Leon, P. Cuesta, T. Rowe, and D.J. Kelvin. 2009. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Critical Care 13: R201.
La Gruta, N.L., K. Kedzierska, J. Stambas, and P.C. Doherty. 2007. A question of self-preservation: immunopathology in influenza virus infection. Immunology and Cell Biology 85: 85–92.
Sirén, J., T. Sareneva, J. Pirhonen, M. Strengell, V. Veckman, I. Julkunen, and S. Matikainen. 2004. Cytokine and contact-dependent activation of natural killer cells by influenza A or Sendai virus-infected macrophages. Journal of General Virology 85: 2357–2364.
Galligan, C.L., T.T. Murooka, R. Rahbar, E. Baig, B. Majchrzak-Kita, and E.N. Fish. 2006. Interferons and viruses: signaling for supremacy. Immunologic Research 35: 27–40.
Billiau, A., H. Heremans, K. Vermeire, and P. Matthys. 2006. Immunomodulatory properties of interferon-gamma. An update. Annals of the New York Academy of Sciences 856: 22–32.
Kaiser, L., R.S. Fritz, S.E. Straus, L. Gubareva, and F.G. Hayden. 2001. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. Journal of Medical Virology 64: 262–268.
Rosenblum, M.D., E. Olasz, J.E. Woodliff, B.D. Johnson, M.C. Konkol, K.A. Gerber, R.J. Orentas, G. Sandford, and R.L. Truitt. 2004. CD200 is a novel p53-target gene involved in apoptosis-associated immune tolerance. Blood 103: 2691–2698.
Snelgrove, R.J., L. Edwards, A.J. Rae, and T. Hussell. 2006. An absence of reactive oxygen species improves the resolution of lung influenza infection. European Journal of Immunology 36: 1364–1373.
Shirey, K.A., W. Lai, A.J. Scott, M. Lipsky, P. Mistry, L.M. Pletneva, C.L. Karp, J. McAlees, T.L. Gioannini, J. Weiss, W.H. Chen, R.K. Ernst, D.P. Rossignol, F. Gusovsky, J.C. Blanco, and S.N. Vogel. 2013. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 497: 498–502.
Acknowledgments
This work was supported by grants from the Shanghai Science and Technology Funds (Nos. 09ZR1403200), National Natural Science Foundation of China (81102359) and the National Science and Technology Major Project for Drug Discovery of the Ministry of Science and Technology of China (Nos. 2011ZX09102-001-27). We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Competing interests
The authors declare they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shi, X., Shi, Z., Huang, H. et al. Ability of Recombinant Human Catalase to Suppress Inflammation of the Murine Lung Induced by Influenza A. Inflammation 37, 809–817 (2014). https://doi.org/10.1007/s10753-013-9800-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9800-2