Abstract
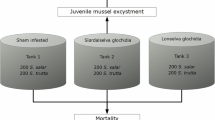
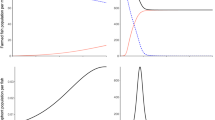
The common duck mussel Anodonta anatina can live in sympatry with—and use the same host, brown trout (Salmo trutta)—as the endangered freshwater pearl mussel Margaritifera margaritifera. Since the glochidia release of A. anatina takes place seasonally earlier than that of M. margaritifera, brown trout can be sequentially exposed first to A. anatina and then to M. margaritifera. Cross-immunity, an immune reaction induced in fish host against glochidia after the infection with glochidia of another mussel species, is possible. Thus, it was studied experimentally if brown trout can be cross immunized against M. margaritifera by earlier infection with A. anatina. In addition, the hypothesis that consecutive exposures of same glochidial species in different years in the same host may create acquired immunity was tested in brown trout against M. margaritifera. Furthermore, the dose dependence of acquired immunity against M. margaritifera glochidia in the Atlantic salmon (S. salar) was also studied. Cross-immunity was not found; suggesting that occurrence of A. anatina does not pose a threat to M. margaritifera. Instead, acquired immunity and its dose dependence were evident, emphasizing the significance of availability of 0+ age group immunologically naïve Atlantic salmon/brown trout for efficient conservation of M. margaritifera.






Similar content being viewed by others
References
Ballesteros, N. A., M. Alonso, S. R. Saint-Jean & S. I. Perez-Prieto, 2015. An oral DNA vaccine against infectious haematopoietic necrosis virus (IHNV) encapsulated in alginate microspheres induces dose-dependent immune responses and significant protection in rainbow trout (Oncorrhynchus mykiss). Fish & Shellfish Immunology 45: 877–888.
Bauer, G., 1987. The parasitic stage of the freshwater pearl mussel (Margaritifera margaritifera L.) II. Susceptibility of brown trout. Archiv für Hydrobiologie 76: 403–412.
Bauer, G. & C. Vogel, 1987. The parasitic stage of the freshwater pearl mussel (Margaritifera margaritifera L.) I. Host response to Glochidiosis. Archiv für Hydrobiologie 76: 393–402.
Bauer, G., S. Hochwald & W. Silkenat, 1991. Spatial distribution of freshwater mussels: the role of host fish and metabolic rate. Freshwater Biology 26: 377–386.
Dodd, B. J., M. C. Barnhart, C. L. Rogers-Lowery, T. B. Fobian & R. V. Ronald Jr., 2005. Cross-resistance of largemouth bass to glochidia of unionid mussels. Journal of Parasitology 91: 1064–1072.
Dodd, B. J., M. C. Barnhart, C. L. Rogers-Lowery, T. B. Fobian & R. V. Ronald Jr., 2006. Persistence of host response against glochidia larvae in Micropterus salmoides. Fish & Shellfish Immunology 21: 473–484.
Douda, K., M. Lopes-Lima, M. Hinzmann, J. Machado, S. Varandas, A. Teixeira & R. Sousa, 2013. Biotic homogenization as a threat to native affiliate species: fish introductions dilute freshwater mussel’s host resources. Diversity and Distributions 19: 933–942.
Eybe, T., F. Thielen, T. Bohn & B. Sures, 2015. Influence of the excystment time on the breeding success of juvenile freshwater pearl mussels (Margaritifera margaritifera). Aquatic Conservation: Marine and Freshwater Ecosystems 25: 21–30.
Geist, J., 2010. Strategies for the conservation of endangered freshwater pearl mussels (Margaritifera margaritifera L.): a synthesis of conservation genetics and ecology. Hydrobiologia 644: 69–88.
Geist, J., M. Porkka & R. Kuehn, 2006. The status of host fish populations and fish species richness in European freshwater pearl mussel (Margaritifera margaritifera L.) streams. Aquatic Conservation: Marine and Freshwater Ecosystems 16: 251–266.
Hastie, L. C. & M. R. Young, 2001. Freshwater pearl mussel (Margaritifera margaritifera) glochidiosis in wild and farmed salmonid stocks in Scotland. Hydrobiologia 445: 109–119.
Helama, S. & I. Valovirta, 2008. The oldest recorded animal in Finland: ontogenetic age and growth in Margaritifera margaritifera (L. 1758) based on internal shell increments. Memoranda Societatis pro Fauna et Flora Fennica 84: 20–30.
Holmes, J. C., 1961. Effects of concurrent infections on Hymenolepis diminuta (Cestoda) and Moniliformis dubius (Acanthocephala). I. General effects and comparison with crowding. Journal of Parasitology 47: 209–216.
Ieshko, E. P., J. Geist, S. A. Murzina, A. E. Veselov, D. I. Lebedeva & V. V. Ziuganov, 2016. The characteristics of the infection of juvenile Atlantic salmon with glochidia of the freshwater pearl mussel in rivers of Northwest Russia. Knowledge & Management of Aquatic Ecosystems 417: 6.
Jackson, J. A., R. J. Pleass, J. Cable, J. E. Bradley & R. C. Tinsley, 2006. Heterogenous interspecific interactions in a host-parasite system. International Journal for Parasitology 36: 1341–1349.
Karvonen, A., O. Seppälä & E. T. Valtonen, 2009. Host immunization shapes interspecific associations in trematode parasites. Journal of Animal Ecology 78: 945–952.
Klemme, I., K. R. Louhi & A. Karvonen, 2016. Host infection history modifies co-infection success of multiple parasite genotypes. Journal of Animal Ecology 85: 591–597.
Hoverman, J. T., B. J. Hoye & P. T. J. Johnson, 2013. Does timing matter? How priority effects influence the outcome of parasite interactions within hosts. Oecologia 173: 1471–1480.
Lello, J., B. Boag, A. Fenton, I. R. Stevenson & P. J. Hudson, 2004. Competition and mutualism among the gut helminths of a mammalian host. Nature 428: 840–844.
Lopes-Lima, M., R. Sousa, J. Geist, D. C. Aldridge, R. Araujo, J. Bergengren, Y. Bespalaya, E. Bódis, L. Burlakova, D. Van Damme, K. Douda, E. Froufe, D. Georgiev, C. Gumpinger, A. Karatayev, Ü. Kebapçi, I. Killeen, J. Lajtner, B. M. Larsen, R. Lauceri, A. Legakis, S. Lois, S. Lundberg, E. Moorkens, G. Motte, K.-O. Nagel, P. Ondina, A. Outeiro, M. Paunovic, V. Prié, T. von Proschwitz, N. Riccardi, M. Rudzīte, M. Rudzītis, C. Scheder, M. Seddon, H. Şereflişan, V. Simić, S. Sokolova, K. Stoeckl, J. Taskinen, A. Teixeira, F. Thielen, T. Trichkova, S. Varandas, H. Vicentini, K. Zajac, T. Zajac & S. Zogaris, 2016. Conservation status of freshwater mussels in Europe: state of the art and future challenges. Biological Reviews. doi:10.1111/brv.12244.
Munang’andu, H. M., B. N. Fredriksen, S. Mutoloki, R. A. Dalmo & Ø. Evensen, 2013. Antigen dose and humoral immune response correspond with protection for inactivated infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salmo salar L.). Veterinary Research 47: 7.
Mutoloki, S., J. B. Jørgensen & Ø. Evensen, 2014. The adaptive immune response in fish. In Gudding, R., A. Lillehaug & Ø. Evensen (eds), Fish Vaccination. Wiley Blackwell, West Sussex: 104–115.
Poulin, R., 2001. Interactions between species and the structure of helminth communities. Parasitology 122: S3–S11.
Read, A. F. & L. H. Taylor, 2001. The ecology of genetically diverse infections. Science 292: 1099–1102.
Rellstab, C., A. Karvonen, K. R. Louhi & J. Jokela, 2013. Genotype-specific vs. cross-reactive host immunity against a macroparasite. PLoS ONE 8: e78427.
Rogers, C. L. & R. V. Dimock Jr., 2003. Acquired resistance of bluegill sunfish Lepomis macrochirus to glochidia larvae of the freshwater mussel Utterbackia imbecillis (Bivalvia: Unionidae) after multiple infections. The Journal of Parasitology 89: 51–56.
Salonen, J. K. & J. Taskinen, 2016. Electrofishing as a new method to search for unknown populations of the endangered freshwater pearl mussel Margaritifera margaritifera. Aquatic Conservation: Marine and Freshwater Ecosystems. doi:10.1002/aqc.2667.
Salonen, J. K., T. J. Marjomäki & J. Taskinen, 2016. An alien fish threatens an endangered parasitic bivalve: the relationship between brook trout (Salvelinus fontinalis) and freshwater pearl mussel (Margaritifera margaritifera) in northern Europe. Marine and Freshwater Ecosystems, Aquatic Conservation. doi:10.1002/aqc.2614.
Taeubert, J. E., B. Gum & J. Geist, 2013. Towards standardization of studies into host relationships of freshwater mussels. Biological Conservation 159: 550–551.
Taskinen, J., T. Mäkelä & E. T. Valtonen, 1997. Exploitation of Anodonta piscinalis (Bivalvia) by trematodes: parasite tactics and host longevity. Annales Zoologici Fennici 34: 37–46.
Treasurer, J. W., L. C. Hastie, D. Hunter, F. Duncan & C. M. Treasurer, 2006. Effects of (Margaritifera margaritifera) glochidial infection on performance of tank-reared Atlantic salmon (Salmo salar). Aquaculture 256: 74–79.
Wächtler, K., M. C. Dreher-Mansur & T. Richter, 2001. Larval types and early postlarval biology in naiads (Unionoida). In Bauer, G. & K. Wächtler (eds), Ecology and Evolution of the Freshwater Mussels Unionoida. Springer, Berlin: 93–125.
Young, M. & J. Williams, 1984. The reproductive biology of the freshwater pearl mussel Margaritifera margaritifera (LINN.) in Scotland II. Laboratory studies. Archiv für Hydrobiologie 100: 29–43.
Ziuganov, V., A. Zotin, L. Nezlin & V. Tretiakov, 1994. The freshwater pearl mussels and their relationships with salmonid fish. Russian Federal Research Institute of Fisheries & Oceanography, Moscow.
Acknowledgements
We are thankful to laboratory technicians of the Konnevesi research station and Tapani Säkkinen for their help in conducting the experiments. We are also grateful to Shariful Islam for proofreading. This research was funded by Maj and Tor Nessling Foundation, Raija and Ossi Tuuliainen Foundation and the EU Interreg IV A Nord Programme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Manuel P. M. Lopes-Lima, Ronaldo G. Sousa, Lyuba E. Burlakova, Alexander Y. Karatayev & Knut Mehler / Ecology and Conservation of Freshwater Bivalves
Rights and permissions
About this article
Cite this article
Chowdhury, M.M.R., Salonen, J.K., Marjomäki, T.J. et al. Interaction between the endangered freshwater pearl mussel Margaritifera margaritifera, the duck mussel Anodonta anatina and the fish host (Salmo): acquired and cross-immunity. Hydrobiologia 810, 273–281 (2018). https://doi.org/10.1007/s10750-017-3114-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3114-6