Abstract
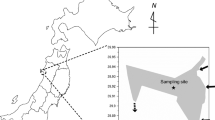
Cyanobacteria blooms pose an increasing threat to ecosystem services. Consequently, understanding their impacts on ecosystem function is important. Cyanobacteria are poor producers of long-chain essential fatty acids (LC-EFA; eicosapentaenoic, docosahexaenoic, and arachidonic acids) and are inadequate for primary consumer growth and reproduction. Higher-level consumers such as planktivorous fishes are hypothesized to be negatively impacted through disruption of LC-EFA availability and transfer up the food web. We tested this hypothesis by comparing fatty acids in yellow perch (Perca flavescens) and white perch (Morone americana) across a gradient of cyanobacteria densities spanning four sites in Lake Champlain and Shelburne Pond, Vermont, USA. Phytoplankton community composition and fatty acid content of seston and fish tissue (liver and muscle) were collected in June, August, and October 2013. Yellow perch liver and muscle tissue increased in percent composition of linoleic acid and α-linolenic acid and decreased in LC-EFA with increased cyanobacteria. Total EFA and arachidonic acid in white perch muscle were negatively related to cyanobacteria. White perch liver did not show any relationship between EFA and cyanobacteria. We conclude that both fish species experienced altered EFA coinciding with cyanobacteria blooms, consistent with disruption of LC-EFA transfer across multiple trophic levels.






Similar content being viewed by others
References
Ahlgren, G., L. Lundstedt, M. Brett & C. Forsberg, 1990. Lipid-composition and food quality of some fresh-water phytoplankton for cladoceran zooplankters. Journal of Plankton Research 12: 809–818.
Ahlgren, G., I. B. Gustafsson & M. Boberg, 1992. Fatty acid content and chemical composition of freshwater microalgae. Journal of Phycology 28: 37–50.
Ahlgren, G., L. Sonesten, M. Boberg & L. Gustafsson, 1996. Fatty acid content of some freshwater fish in lakes of different trophic levels – a bottom up effect? Ecology of Freshwater Fish 5: 15–27.
Aitchison, J., 1986. The statistical analysis of compositional data. Chapman and Hall, London.
Bec, A., M. E. Perga, C. Desvilettes & G. Bourdier, 2010. How well can the fatty acid content of lake seston be predicted from its taxonomic composition? Freshwater Biology 55: 1958–1972.
Benjamini, Y. & Y. Hochberg, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 57(1): 289–300.
Bligh, E. G. & W. J. Dyer, 1959. A rapid method for total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37: 911–917.
Brett, M. T. & D. C. Müller-Navarra, 1997. The role of highly unsaturated fatty acids in aquatic food web processes. Freshwater Biology 38: 483–499.
Brookes, J. D. & C. Carey, 2011. Resilience to blooms. Science 334: 46–47.
Budge, S. M., S. J. Iverson, W. D. Bowen & R. G. Ackman, 2002. Among and within-species variability in fatty acid signatures of marine fish and invertebrates on the Scotian Shelf, Georges Bank, and southern Gulf of St. Lawrence. Canadian Journal of Fisheries and aquatic sciences 59: 886–898.
Buskey, E. J., 2008. How does eutrophication affect the role of grazers in harmful algal bloom dynamics? Harmful Algae 8: 152–157.
Couture, S. C. & M. C. Watzin, 2008. Diet of invasive adult white perch (Morone americana) and their effects on the zooplankton community in Missisquoi Bay, Lake Champlain. Journal of Great Lakes Research 34: 485–494.
Christie, W. W., 1989. Gas chromatography and lipids, Vol. 39. Oily Press, Ayr: 37–38.
Czesny, S. J., J. Rinchard, S. D. Hanson, J. M. Dettmers & K. Dabrowski, 2011. Fatty acid signatures of Lake Michigan prey fish and invertebrates: among-species differences and spatiotemporal variability. Canadian Journal of Fisheries and Aquatic Sciences 68: 1211–1230.
Demott, W. & D. C. Müller-Navarra, 1997. The importance of highly unsaturated fatty acids in zooplankton nutrition: evidence from experiments with Daphnia, a cyanobacterium and lipid emulsions. Freshwater Biology 38: 649–664.
Elliott, J. A., 2010. The seasonal sensitivity of cyanobacteria and other phytoplankton to changes in flushing rate and water temperature. Global Change Biology 16: 864–876.
Facey, D. E., J. E. Marsden, T. B. Mihuc & E. A. Howe, 2012. Lake Champlain 2010: a summary of recent research and monitoring initiatives. Journal of Great Lakes ResearchSupplement 38(Supplement 1): 1–5.
Ferber, L. R., S. N. Levine, A. Lini & G. P. Livingston, 2004. Do cyanobacteria dominate in eutrophic lakes because they fix atmospheric nitrogen? Freshwater Biology 49: 690–708.
Galloway, A. W. & M. Winder, 2015. Partitioning the relative importance of phylogeny and environmental conditions on phytoplankton fatty acids. PLoS One 10(6): e0130053.
Gladyshev, M. I., N. N. Sushchik, A. A. Kolmakova, G. S. Kalachova, E. S. Kravchuk, E. A. Ivanova & O. N. Makhutova, 2007. Seasonal correlations of elemental and ω3 PUFA composition of seston and dominant phytoplankton species in a eutrophic Siberian Reservoir. Aquatic Ecology 41: 9–23.
Gladyshev, M. I., N. N. Sushchik, O. N. Makhutova, O. P. Dubovskaya, E. S. Kravchuk, G. S. Kalachova & E. B. Khromechek, 2010. Correlations between fatty acid composition of seston and zooplankton and effects of environmental parameters in a eutrophic Siberian reservoir. Limnologica 40: 343–357.
Graeb, B. D., J. M. Dettmers, D. H. Wahl & C. E. Cáceres, 2004. Fish size and prey availability affect growth, survival, prey selection, and foraging behavior of larval yellow perch. Transactions of the American Fisheries Society 133: 504–514.
Happel, A., S. Creque, J. Rinchard, T. Höök, H. Bootsma, J. Janssen, D. Jude & S. Czesny, 2015. Exploring yellow perch diets in Lake Michigan through stomach content, fatty acids, and stable isotope ratios. Journal of Great Lakes Research. doi:10.1016/j.jglr.2015.03.025.
Jöhnk, K. D., J. Huisman, J. Sharples, B. Sommeijer, P. M. Visser & J. M. Stroom, 2008. Summer heat waves promote blooms of harmful cyanobacteria. Global Change Biology 14: 495–512.
Keast, A., 1977. Diet overlaps and feeding relationships between the year classes in the yellow perch (Perca flavescens). Environmental Biology of Fishes 2: 53–70.
Lane, R. L., J. T. Trushenski & C. C. Kohler, 2006. Modification of fillet composition and evidence of differential fatty acid turnover in sunshine bass Morone chrysops × M. saxatilis following change in dietary lipid source. Lipids 41: 1029–1038.
Léveillé, J. C., C. Amblard & G. Bourdier, 1997. Fatty acids as specific algal markers in a natural lacustrian phytoplankton. Journal of Plankton Research 19: 469–490.
Limburg, K. E., M. L. Pace, D. Fischer & K. K. Arend, 1997. Consumption, selectivity, and use of zooplankton by larval striped bass and white perch in a seasonally pulsed estuary. Transactions of the American Fisheries Society 126: 607–621.
Los, D. A. & K. S. Mironov, 2015. Modes of fatty acid saturation in cyanobacteria: an update. Life 5: 554–567.
Marsden, J. E. & M. Hauser, 2009. Exotic species in Lake Champlain. Journal of Great Lakes Research 35: 250–265.
Martin-Creuzburg, D., E. von Elert & K. H. Hoffmann, 2008. Nutritional constraints at the cyanobacteria–Daphnia magna interface: the role of sterols. Limnology and Oceanography 53: 456–468.
Mjoun, K., K. A. Rosentrater & M. L. Brown, 2012. Culture performance and tissue fatty acid compositions of yellow perch (Perca flavescens) fed different dietary lipids. Aquaculture 360: 17–24.
Müller-Navarra, D. C., M. T. Brett, A. M. Liston & C. R. Goldman, 2000. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 403: 74–77.
Müller-Navarra, D. C., M. T. Brett, S. Park, S. Chandra, A. P. Ballantyne, E. Zorita & C. R. Goldman, 2004. Unsaturated fatty acid content in seston and tropho-dynamic coupling in lakes. Nature 427: 69–72.
Nematipour, G. R. & D. M. Gatlin 3rd, 1993. Requirement of hybrid striped bass for dietary (n-3) highly unsaturated fatty acids. The Journal of Nutrition 123: 744–753.
Nuernberg, K., D. Dannenberger, K. Ender & G. Nuernberg, 2007. Comparison of different methylation methods for the analysis of conjugated linoleic acid isomers by silver ion HPLC in beef lipids. Journal of Agricultural and Food Chemistry 55: 598–602.
Oksanen, J. G.F Blanchet, R. Kindt, P Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens & H.Wagner (2016). vegan: Community Ecology Package. R package version 2.3-3. http://CRAN.R-project.org/package=vegan.
O’Neil, J., T. W. Davis, M. A. Burford & C. Gobler, 2012. The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae 14: 313–334.
Paerl, H. W. & J. Huisman, 2008. Climate – blooms like it hot. Science 320: 57–58.
Perga, M. E., A. Bec & O. Anneville, 2009. Origins of carbon sustaining the growth of whitefish Coregonus lavaretus early larval states in Lake Annecy: insights from fatty–acid biomarkers. Journal of Fish Biology 74: 2–17.
Perga, M. E., I. Domaizon, J. Guillard, V. Hamelet & O. Anneville, 2013. Are cyanobacterial blooms trophic dead ends? Oecologia 172: 551–562.
R Development Core Team, 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.
Ravet, J. L., M. T. Brett & G. B. Arhonditsis, 2010. The effects of seston lipids on zooplankton fatty acid composition in Lake Washington, Washington, USA. Ecology 91: 180–190.
Sargent, J., J. Bell, M. Bell, R. Henderson & D. Tocher, 1993. The metabolism of phospholipids and polyunsaturated fatty acids in fish. Coastal and Estuarine Studies 43: 103–124.
Sargent, J., L. McEvoy, A. Estevez, G. Bell, M. Bell, J. Henderson & D. Tocher, 1999. Lipid nutrition of marine fish during early development: current status and future directions. Aquaculture 179: 217–229.
Smeltzer, E., A. D. Shambaugh & P. Stangel, 2012. Environmental change in Lake Champlain revealed by long-term monitoring. Journal of Great Lakes Research 38: 6–18.
Strandberg, U., S. J. Taipale, M. Hiltunen, A. W. E. Galloway, M. T. Brett & P. Kankaala, 2015. Inferring phytoplankton composition with a fatty acid modeling. Ecosphere 6: 1–18.
Tidwell, J. H., S. D. Coyle, J. Evans, C. Weibel, J. McKinney, K. Dodson & H. Jones, 1999. Effect of culture temperature on growth, survival, and biochemical composition of yellow perch Perca fiavescens. Journal of the World Aquaculture Society 30: 324–330.
Tocher, D. R., 2010. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquaculture Research 41: 717–732.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen phytoplankton: methodik. Mitteilungen der internationale Vereinigung für theoretische und angewandte Limnologie 9: 1–38.
Vanni, M. J. & D. L. Findlay, 1990. Trophic cascades and phytoplankton community structure. Ecology 71: 921–937.
Vermont Department of Environmental Conservation (VTDEC) and New York State Department of Environmental Conservation (NYDEC). 2013. Long-term water quality and biological monitoring project for Lake Champlain Quality Assurance Project Plan. Grand Isle, Vermont. Available from http://www.watershedmanage-ment.vt.gov/lakes/htm/lp_longterm.htm.
Xu, X. & P. Kestemont, 2002. Lipid metabolism and FA composition in tissues of Eurasian perch Perca fluviatilis as influenced by dietary fats. Lipids 37: 297–304.
Wetzel, R. G., 2012. Lipids in freshwater ecosystems. In Arts, M. T. & B. C. Wainmann (eds). Springer Science & Business Media.
Acknowledgments
Fish samples were collected in accordance with University of Vermont Institutional Animal Care and Use Committee guidelines (IACUC 13-051). We thank Melissa Bainbridge, Katie Bryan, Emily Berry, and Nora Hill for their assistance with sample collection and preparation. We would like to thank Captain Steve Cluett of the RV Melosira for always going the extra step and to make sure sample collections were as successful as possible. Support provided by Vermont EPSCoR with funds from the National Science Foundation (NSF) Grant EPS-1101317, a NSF Research Experiences for Undergraduates award to EN (Award DBI-1358838), and a University of Vermont Office of Undergraduate Research Summer Mini-Grant to KR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gearhart, T.A., Ritchie, K., Nathan, E. et al. Alteration of essential fatty acids in secondary consumers across a gradient of cyanobacteria. Hydrobiologia 784, 155–170 (2017). https://doi.org/10.1007/s10750-016-2864-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2864-x