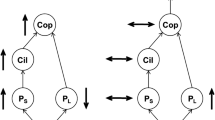
Abstract
Copepods, the most abundant planktonic metazoans, constitute an intermediate trophic position between phytoplankton and higher trophic-level animals such as fish and jellyfish. Fish and jellyfish are adversaries because they often compete for prey copepods and also can be prey of each other. The classical food chain represented by phytoplankton–copepod–fish is the main process leading to efficient and sustainable production of fish as human food. At present, more than 75% of world fish stocks are fully or over exploited. On the other hand, jellyfish populations have increased world-wide, particularly in waters under significant human influences. Two such cases are seen in East Asian waters, where massive blooms of moon jellyfish (Aurelia aurita s.l.) and giant jellyfish (Nemopilema nomurai) have repeatedly occurred in recent decades, causing severe damage to local fisheries. In this article, I will review the pivotal role of copepods in marine ecosystems, particularly in the Inland Sea of Japan, where the annual fish catch per unit area is among the world’s highest. Then, I will describe an ongoing ecosystem shift from dominance by fish to dominance by jellyfish as a consequence of human forcing. Finally, I will propose to create “sato-umi”, a coastal sea with high productivity and biodiversity with wise human interaction, where copepod production would most efficiently transforms into food for humans.










Similar content being viewed by others
References
Aoyama, M., S. Uye & H. Takeoka, 2005. Annual variations in the occurrence of the scyphomedusae Aurelia aurita population in Uwa Sea, western Shikoku, and their predation impact on mesozooplankton. Bulletin of Plankton Society of Japan 52: 21–25. (in Japanese with English abstract).
Arai, M. N., 2001. Pelagic coelenterates and eutrophication: a review. Hydrobiologia 451: 69–87.
Attrill, M. J., J. Wright & M. Edwards, 2007. Climate-related increases in jellyfish frequency suggest a more gelatinous future for the North Sea. Limnology and Oceanography 52: 480–485.
Azam, F., T. Fenchel, J. G. Field, J. S. Gray, L. A. Meyer-Reil & F. Thingstad, 1983. The ecological role of water-column microbes in the sea. Marine Ecology Progress Series 10: 257–263.
Bernard, C. & F. Rassoulzadegan, 1990. Bacteria or microflagellates as a major food source for marine ciliates: possible implications for the microzooplankton. Marine Ecology Progress Series 64: 147–155.
Brewer, R. H., 1978. Larval settlement behavior in the jellyfish Aurelia aurita (Linnaeus) (Scyphozoa: Semaeostomeae). Estuaries 1: 120–122.
Brodeur, R. D., H. Sugisaki & G. L. Hunt Jr., 2002. Increases in jellyfish biomass in the Bering Sea: implication for the ecosystem. Marine Ecology Progress Series 233: 89–103.
Brodeur, R. D., M. B. Decker, L. Ciannelli, J. E. Purcell, N. A. Bond, P. J. Stabeno, G. L. Hunt Jr. & E. Acuna, 2008. Rise and fall of jellyfish in the Bering Sea in relation to climate regime shifts. Progress of Oceanography 77: 103–111.
Chang, P. H. & A. Isobe, 2003. A numerical study on the Changjiang diluted water in the Yellow and East China Seas. Journal of Geophysical Research C 108: 3299.
Condon, R. H., M. B. Decker & J. E. Purcell, 2001. Effects of low dissolved oxygen on survival and asexual reproduction of scyphozoan polyps (Chrysaora quinquecirrha). Hydrobiologia 451: 89–95.
Cushing, D. H., 1976. Biology of fisheries in the pelagic community. In Cusing, D. H. & J. J. Walsh (eds), The Ecology of the Sea. Blackwell, Oxford: 317–340.
Endo, T., 1970. On primary production in the Seto Inland Sea. Journal of Faculty of Fisheries and Animal Husbandry, Hiroshima University 9: 177–221. (in Japanese with English abstract).
Graham, W. M., 2001. Numerical increase and distribution shifts of Chrysaora quinquecirrha (Desor) and Aurelia aurita (Linné) (Cnidaria: Scyphozoa) in the northern Gulf of Mexico. Hydrobiologia 451: 97–111.
Hardy, A. C., 1956. The Open Sea, its Natural History: The World of Plankton. Collins, London.
Humborg, C., V. Ittekkot, A. Cociasu & B. Bodungen, 1997. Effects of Danube River dam on Black Sea biogeochemistry and ecosystem structure. Nature 386: 385–388.
Ishii, H., 2001. The influence of environmental changes upon the coastal plankton ecosystems, with special reference to mass occurrence of jellyfish. Bulletin of the Plankton Society Japan 48: 55–61. (in Japanese with English abstract).
Ishii, H., T. Ohba & T. Kobayashi, 2008. Effects of low dissolved oxygen on planura settlement, polyp growth and asexual reproduction of Aurelia aurita. Plankton and Benthos Research 3(Suppl.): 107–113.
Kawahara, M., S. Uye, K. Ohtsu & H. Iizumi, 2006. Unusual population explosion of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in East Asian waters. Marine Ecology Progress Series 307: 161–173.
Ketchum, B. H. (ed.), 1983. Estuaries and Enclosed Seas. Elsevier, Amsterdam.
Ki, J.-S., D.-S. Hwang, K. Shin, W. D. Yoon, D. Lim, Y. S. Kang, Y. Lee & J.-S. Lee, 2008. Recent moon jelly (Aurelia sp. 1) blooms in Korean coastal waters suggest global expansion: examples inferred from mitochondrial COI and nuclear ITS-5/8S rNDA sequences. ICES Journal of Marine Science 65: 443–452.
Kishinouye, K., 1922. Echizen kurage (Nemopilema nomurai). Dobutsugaku Zasshi 34: 343–345. (in Japanese).
Kuwabara, R., S. Sato & N. Noguchi, 1969. Ecological studies on the medusa, Aurelia aurita. 1. Distribution of Aurelia patches in the north-eastern region of Tokyo Bay in summer of 1966 and 1967. Bulletin of the Japanese Society of Scientific Fisheries 35: 156–162. (in Japanese with English abstract).
Liang, D. & S. Uye, 1996a. Population dynamics and production of the planktonic copepods in a eutrophic inlet of the Inland Sea of Japan. II. Acartia omorii. Marine Biology 125: 109–117.
Liang, D. & S. Uye, 1996b. Population dynamics and production of the planktonic copepods in a eutrophic inlet of the Inland Sea of Japan. III. Paracalanus sp. Marine Biology 127: 219–227.
Liang, D. & S. Uye, 1997a. Seasonal reproductive biology of the egg-carrying calanoid copepod Pseudodiaptomus marinus in a eutrophic inlet of the Inland Sea of Japan. Marine Biology 128: 409–414.
Liang, D. & S. Uye, 1997b. Population dynamics and production of the planktonic copepods in a eutrophic inlet of the Inland Sea of Japan. IV. Pseudodiaptomus marinus, the egg-carrying calanoid. Marine Biology 128: 415–421.
Liang, D., S. Uye & T. Onbe, 1996. Population dynamics and production of the planktonic copepods in a eutrophic inlet of the Inland Sea of Japan. I. Centropages abdominalis. Marine Biology 124: 527–536.
Lin, C., J. Ning, J. Su, Y. Lin & B. Xu, 2005. Environmental changes and the responses of the ecosystem of the Yellow Sea during 1976–2000. Journal of Marine Systems 55: 223–234.
Lynam, C. P., S. J. Hay & A. S. Brierley, 2004. Interannual variability in abundance of North Sea jellyfish and links to the North Atlantic Oscillation. Limnology and Oceanography 49: 637–643.
Lynam, C. P., M. J. Gibbon, B. E. Axelsen, C. A. J. Sparks, J. Coetzee, B. G. Heywood & A. S. Brierley, 2006. Jellyfish overtake fish in a heavily fished ecosystem. Current Biology 16: R492–R493.
Okaichi, T. & T. Yanagi, 1997. Sustainable Development in the Seto Inland Sea, Japan: From the Viewpoint of Fisheries. Terrapub, Tokyo.
Omori, M. & M. Kitamura, 2004. Taxonomic review of three Japanese species of edible jellyfish (Schyphozoa: Rhizostomeae). Plankton Biology and Ecology 51: 36–51.
Omori, M., H. Ishii & A. Fujinaga, 1995. Life history strategy of Aurelia aurita (Cnidaria, Scyphomedusae) and its impact on the zooplankton community of Tokyo Bay. ICES Journal of Marine Science 52: 597–603.
Pagés, F., 2001. Past and present anthropogenic factors promoting the invasion, colonization and dominance by jellyfish of a Spanish coastal lagoon. In Gelatinous Zooplankton Outbreaks: Theory and Practice. IESM Workshop Series 14, Monaco: 69–71.
Parsons, T. R. & C. M. Lalli, 2002. Jellyfish population explosions: Revisiting a hypothesis of possible causes. La Mer 40: 111–121.
Pomeroy, L. R., 1974. The ocean’s food web, a changing paradigm. BioScience 24: 499–504.
Postma, H. & J. J. Zijlstra (eds), 1988. Continental Shelves. Elsevier, Amsterdam.
Purcell, J. E., 2005. Climate effects on formation of jellyfish and ctenophore blooms. Journal of Marine Biological Association of the United Kingdom 85: 461–476.
Purcell, J. E., S. Uye & W.-T. Lo, 2007. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series 350: 153–174.
Rabalais, N. N. & R. E. Turner (eds), 1998. Coastal Hypoxia: Consequences for Living Resources and Ecosystems. American Geophysical Union, Washington, DC.
Reizen, N. & A. Isobe, 2006. Numerical tracer experiments representing behavior of the giant jellyfish, Nemopilema nomurai, in the Yellow and East China Seas. Oceanography in Japan 15: 425–436. (in Japanese with English abstract).
Rutherford Jr., L. D. & E. V. Thusen, 2005. Metabolic performance and survival of medusae in estuarine hypoxia. Marine Ecology Progress Series 294: 189–200.
Ryther, J. H., 1969. Photosynthesis and fish production in the sea. The production of organic matter and its conversion to higher forms of life vary throughout the world ocean. Science 166: 72–76.
Sanders, R. W., D. A. Caron & U.-G. Berninger, 1992. Relationship between bacteria and heterotrophic nanoplankton in marine and fresh waters: an inter-ecosystem comparison. Marine Ecology Progress Series 86: 1–14.
Shimomura, T., 1959. On the unprecedented flourishing of ‘Echizen Kurage’ Stomolophus nomurai (Kishinouye), in the Tsushima Current regions in autumn, 1958. Bulletin of the Japan Sea Regional Fisheries Research Laboratory 7: 85–107. (In Japanese with English abstract).
Shoji, J., R. Masuda, Y. Yamashita & M. Tanaka, 2005. Effects of low dissolved oxygen concentrations on behavior and predation rate on fish larvae by moon jellyfish Aurelia aurita and by juvenile Spanish mackerel Scomberomorus niphonius. Marine Biology 147: 863–868.
Takahashi, S. & S. Seiki, 2004. Long-term change of water temperature in the Seto Inland Sea. Sea and Sky 80: 11–16. (in Japanese with English abstract).
Takeoka, H., 1997. Comparison of the Seto Inland Sea with other enclosed seas from around the world. In Okaichi, T. & T. Yanagi (eds), Sustainable Development in the Seto Inland Sea, Japan: From the Viewpoint of Fisheries. Terrapub, Tokyo: 223–247.
Takikawa, T., J. H. Yoon & K. D. Cho, 2005. The Tsushima warm current through Tsushima Straits estimated from ferryboat ADCP data. Journal of Physical Oceanography 35: 1154–1168.
Tang, Q., X. Jin, J. Wang, Z. Zhuang, Y. Cui & T. Meng, 2003. Decadal-scale variations of ecosystem productivity and control mechanisms in the Bohai Sea. Fisheries Oceanography 12: 223–233.
Toyokawa, M., T. Furota & M. Terazaki, 2000. Life history and seasonal abundance of Aurelia aurita medusae in Tokyo Bay, Japan. Plankton Biology and Ecology 47: 48–58.
Turner, J. T. & P. A. Tester, 1997. Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnology and Oceanography 42: 1203–1214.
Unoki, S. & M. Kishino, 1977. Average ocean condition and water exchange in Tokyo Bay. Technical Report of Physical Oceanography Laboratory, Institute of Physics and Chemistry Research 1: 1–89. (in Japanese).
Uotani, I., A. Izuha & K. Asai, 1978. Food habits and selective feeding of anchovy larvae (Engraulis japonicus). Bulletin of the Japanese Society of Scientific Fisheries 44: 427–434. (in Japanese with English abstract).
Uye, S., 1982. Length-weight relationships of important zooplankton from the Inland Sea of Japan. Journal of Oceanographic Society of Japan 38: 149–158.
Uye, S., 1988. Temperature-dependent development and growth of Calanus sinicus (Copepoda: Calanoida) in the laboratory. Hydrobiologia 167(168): 285–293.
Uye, S., 1991. Temperature-dependent development and growth of the planktonic copepod Paracalanus sp. in the laboratory. Bulletin of the Plankton Society of Japan Special Volume: 627–636.
Uye, S., 1994. Replacement of large copepods by small ones with eutrophication of embayments: cause and consequence. Hydrobiologia 292(293): 513–519.
Uye, S., 2005a. Recent jellyfish bloom in the coastal waters of the East Asia: cause and consequence. Bulletin of Coastal Oceanography 43: 13–17. (in Japanese with English abstract).
Uye, S., 2005b. Growth and production of planktonic copepods: a key component to support fish production. In Nagasawa, K. (ed.), Introduction to Copepodology. Tokai University Press, Tokyo: 72–85. in Japanese.
Uye, S., 2008. Blooms of the giant jellyfish Nemopilema nomurai: a threat to the fisheries sustainability of the East Asian Marginal Seas. Plankton and Benthos Research 3(Suppl): 125–131.
Uye, S. & K. Sano, 1995. Seasonal reproductive biology of the small cyclopoid copepod Oithona davisae in a temperate eutrophic inlet. Marine Ecology Progress Series 118: 121–128.
Uye, S. & K. Sano, 1998. Seasonal variations in biomass, growth rate and production rate of the small cyclopoid copepod Oithona davisae in a temperate eutrophic inlet. Marine Ecology Progress Series 163: 37–44.
Uye, S. & H. Shimauchi, 2005. Population biomass, feeding, respiration and growth rates, and carbon budge of the scyphomedusa Aurelia aurita in the Inland Sea of Japan. Journal of Plankton Research 27: 237–248.
Uye, S. & T. Shimazu, 1997. Geographical and seasonal variations in abundance, biomass and estimated production rates of meso- and macrozooplankton in the Inland Sea of Japan. Journal of Oceanography 53: 529–538.
Uye, S. & K. Takamatsu, 1990. Feeding interactions between planktonic copepods and red-tide flagellates from Japanese coastal waters. Marine Ecology Progress Series 59: 97–107.
Uye, S. & Y. Ueta, 2004. Recent increase of jellyfish populations and their nuisance to fisheries in the Inland Sea of Japan. Bulletin of Japanese Society of Fisheries Oceanography 68: 9–19. (in Japanese with English abstract).
Uye, S., N. Nagano & H. Tamaki, 1996. Geographical and seasonal variations in abundance, biomass and estimated production rates of microzooplankton in the Inland Sea of Japan. Journal of Oceanography 52: 689–703.
Uye, S., N. Iwamoto, T. Ueda, H. Tamaki & K. Nakahira, 1999. Geographical variations in the trophic structure of the plankton community along a eutrophic–mesotrophic–oligotrophic transect. Fisheries Oceanography 8: 227–237.
Uye, S., N. Fujii & H. Takeoka, 2003. Unusual aggregations of the scyphomedusa Aurelia aurita in coastal waters along western Shikoku, Japan. Plankton Biology and Ecology 50: 17–21.
Wang, B., 2006. Cultural eutrophication in the Changjiang (Yangtze River) plume: history and perspective. Estuarine Coastal and Shelf Science 69: 471–477.
Watanabe, T. & H. Ishii, 2001. In situ estimation of ephyrae liberated from polyps of Aurelia aurita using settling plates in Tokyo Bay, Japan. Hydrobiologia 451: 247–258.
Yanagi, T., 2007. Sato-Umi: A New Concept for Coastal Sea Management. Terrapub, Tokyo.
Yasuda, T., 2004. On the unusual occurrence of the giant medusa Nemopilema nomurai in Japanese waters. Nippon Suisan Gakkaishi 70: 380–386. (in Japanese).
Yoon, W. D., J.-Y. Yang, M. B. Shim & H.-K. Kang, 2008. Physical processes influencing the occurrence of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) around Jeju Island, Korea. Journal of Plankton Research 30: 251–260.
Zaitsev, Y. P., 1992. Recent changes in the trophic structure of the Black Sea. Fisheries Oceanography 2: 108–189.
Acknowledgments
I entered the world of copepodology (e.g., on copepod resting eggs) by guidance of Dr. T. Onbe when I was a senior of Hiroshima University, and was enabled to widen my interest in biological oceanography (e.g., copepod production ecology) by advice of late Drs. A. Fleminger and M. M. Mullin when I was a visiting student at Scripps Institution of Oceanography. Since I became a faculty member of Hiroshima University, so many people have supported my work. I thank the captain and crew of the T&R/V Toyoshio Maru, Hiroshima University, for their tireless support at sea. My gratitude is extended to staff and students of my laboratory for assistance and collaboration. My jellyfish study was partially supported by research grants from the Japan Society for the Promotion of Science and from the Ministry of Agriculture, Forestry and Fisheries. This article was presented as Maxilliped Lecture at the 10th International Conference on Copepoda in Pattaya, Thailand, July 2008. English of the early manuscript was edited by Sea Pen Scientific Writing, LLC. Constructive comments from three anonymous reviewers are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: L. Sanoamuang & J. S. Hwang / Copepoda: Biology and Ecology
Electronic supplementary material
Below is the link to the electronic supplementary material.
10750_2010_208_MOESM1_ESM.pdf
Appendix 1. Bird-eye view of white carpet-like aggregation of Aurelia aurita in Uwa Sea, western Shikoku, in August, 2000 (from Uye et al., 2003) (111 kb)
10750_2010_208_MOESM2_ESM.pdf
Appendix 2. Medusae of Nemopilema nomurai trapped in a set-net in Iwate Prefecture in December, 2005 (from Uye, 2008) (857 kb)
Rights and permissions
About this article
Cite this article
Uye, Si. Human forcing of the copepod–fish–jellyfish triangular trophic relationship. Hydrobiologia 666, 71–83 (2011). https://doi.org/10.1007/s10750-010-0208-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0208-9