Abstract
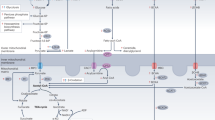
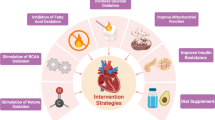
This review article offers a detailed examination of metabolic adaptations in pressure overload hypertrophic hearts, a condition that plays a pivotal role in the progression of heart failure with preserved ejection fraction (HFpEF) to heart failure with reduced ejection fraction (HFrEF). The paper delves into the complex interplay between various metabolic pathways, including glucose metabolism, fatty acid metabolism, branched-chain amino acid metabolism, and ketone body metabolism. In-depth insights into the shifts in substrate utilization, the role of different transporter proteins, and the potential impact of hypoxia-induced injuries are discussed. Furthermore, potential therapeutic targets and strategies that could minimize myocardial injury and promote cardiac recovery in the context of pressure overload hypertrophy (POH) are examined. This work aims to contribute to a better understanding of metabolic adaptations in POH, highlighting the need for further research on potential therapeutic applications.


Similar content being viewed by others
Availability of data and materials
This is not applicable.
References
Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS (2023) Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res 118:3272–3287. https://doi.org/10.1093/cvr/cvac013
Gibb AA, Hill BG (2018) Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res 123:107–128. https://doi.org/10.1161/CIRCRESAHA.118.312017
Savarese G, Lund LH (2017) Global public health burden of heart failure. Card Fail Rev 3:7–11. https://doi.org/10.15420/cfr.2016:25:2
Redfield MM, Borlaug BA (2023) Heart failure with preserved ejection fraction: a review. JAMA 329:827–838. https://doi.org/10.1001/jama.2023.2020
Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED (2021) Cardiac energy metabolism in heart failure. Circ Res 128:1487–1513. https://doi.org/10.1161/CIRCRESAHA.121.318241
Ritterhoff J, Tian R (2023) Metabolic mechanisms in physiological and pathological cardiac hypertrophy: new paradigms and challenges. Nat Rev Cardiol. https://doi.org/10.1038/s41569-023-00887-x
Ritterhoff J, Tian R (2017) Metabolism in cardiomyopathy: every substrate matters. Cardiovasc Res 113:411–421. https://doi.org/10.1093/cvr/cvx017
Ho KL, Karwi QG, Wagg C, Zhang L, Vo K, Altamimi T, Uddin GM, Ussher JR, Lopaschuk GD (2021) Ketones can become the major fuel source for the heart but do not increase cardiac efficiency. Cardiovasc Res 117:1178–1187. https://doi.org/10.1093/cvr/cvaa143
Kolwicz SC, Purohit S Jr, Tian R (2013) Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res 113:603–616. https://doi.org/10.1161/CIRCRESAHA.113.302095
Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Z, Yanxiang GJ, White E, Rabinowitz JD (2017) Glucose feeds the TCA cycle via circulating lactate. Nature 551:115–118. https://doi.org/10.1038/nature24057
Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, Rabinowitz JD, Frankel DS, Arany Z (2020) Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 370:364–368. https://doi.org/10.1126/science.abc8861
Taegtmeyer H, Wilson CR, Razeghi P, Sharma S (2005) Metabolic energetics and genetics in the heart. Ann N Y Acad Sci 1047:208–218. https://doi.org/10.1196/annals.1341.019
De Jong KA, Lopaschuk GD (2017) Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol 33:860–871. https://doi.org/10.1016/j.cjca.2017.03.009
Chen Z, Jin ZX, Cai J, Li R, Deng KQ, Ji YX, Lei F, Li HP, Lu Z, Li H (2022) Energy substrate metabolism and oxidative stress in metabolic cardiomyopathy. J Mol Med 100:1721–1739. https://doi.org/10.1007/s00109-022-02269-1
Karwi QG, Uddin GM, Ho KL, Lopaschuk GD (2018) Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med 5:68. https://doi.org/10.3389/fcvm.2018.00068
Dugani CB, Klip A (2005) Glucose transporter 4: cycling, compartments and controversies. EMBO Rep 6:1137–1142. https://doi.org/10.1038/sj.embor.7400584
Kutsche HS, Schreckenberg R, Weber M, Hirschhauser C, Rohrbach S, Li L, Niemann B, Schulz R, Schluter KD (2020) Alterations in Glucose metabolism during the transition to heart failure: the contribution of UCP-2. Cells 9. https://doi.org/10.3390/cells9030552
Hua Y, Zhang Y, Ren J (2012) IGF-1 deficiency resists cardiac hypertrophy and myocardial contractile dysfunction: role of microRNA-1 and microRNA-133a. J Cell Mol Med 16:83–95. https://doi.org/10.1111/j.1582-4934.2011.01307.x
Paternostro G, Clarke K, Heath J, Seymour AM, Radda GK (1995) Decreased GLUT-4 mRNA content and insulin-sensitive deoxyglucose uptake show insulin resistance in the hypertensive rat heart. Cardiovasc Res 30:205–211
Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H (2001) Metabolic gene expression in fetal and failing human heart. Circulation 104:2923–2931. https://doi.org/10.1161/hc4901.100526
Paternostro G, Pagano D, Gnecchi-Ruscone T, Bonser RS, Camici PG (1999) Insulin resistance in patients with cardiac hypertrophy. Cardiovasc Res 42:246–253. https://doi.org/10.1016/s0008-6363(98)00233-8
van Gerwen J, Shun-Shion AS, Fazakerley DJ (2023) Insulin signalling and GLUT4 trafficking in insulin resistance. Biochem Soc Trans 51:1057–1069. https://doi.org/10.1042/BST20221066
Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD (2013) Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Fail 6:1039–1048. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000228
Riehle C, Abel ED (2016) Insulin signaling and heart failure. Circ Res 118:1151–1169. https://doi.org/10.1161/CIRCRESAHA.116.306206
Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS, Kahn BB (1999) Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Investig 104:1703–1714. https://doi.org/10.1172/JCI7605
Wende AR, Kim J, Holland WL, Wayment BE, O’Neill BT, Tuinei J, Brahma MK, Pepin ME, McCrory MA, Luptak I, Halade GV, Litwin SE, Abel ED (2017) Glucose transporter 4-deficient hearts develop maladaptive hypertrophy in response to physiological or pathological stresses. Am J Physiol Heart Circ Physiol 313:H1098–H1108. https://doi.org/10.1152/ajpheart.00101.2017
Belke DD, Larsen TS, Gibbs EM, Severson DL (2001) Glucose metabolism in perfused mouse hearts overexpressing human GLUT-4 glucose transporter. Am J Physiol Endocrinol Metab 280:E420-427. https://doi.org/10.1152/ajpendo.2001.280.3.E420
Hansen PA, Gulve EA, Marshall BA, Gao J, Pessin JE, Holloszy JO, Mueckler M (1995) Skeletal muscle glucose transport and metabolism are enhanced in transgenic mice overexpressing the Glut4 glucose transporter. J Biol Chem 270:1679–1684. https://doi.org/10.1074/jbc.270.5.1679
Li J, Hu X, Selvakumar P, 3rd Russell RR, Cushman SW, Holman GD, Young LH (2004) Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am J Physiol Endocrinol Metab 287:E834-841. https://doi.org/10.1152/ajpendo.00234.2004
3rd Russel RR, Bergeron R, Shulman GI, Young LH (1999) Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol 277:H643-649. https://doi.org/10.1152/ajpheart.1999.277.2.H643
Tian R, Musi N, D’Agostino J, Hirshman MF, Goodyear LJ (2001) Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation 104:1664–1669. https://doi.org/10.1161/hc4001.097183
Kim M, Tian R (2011) Targeting AMPK for cardiac protection: opportunities and challenges. J Mol Cell Cardiol 51:548–553. https://doi.org/10.1016/j.yjmcc.2010.12.004
Lund S, Holman GD, Schmitz O, Pedersen O (1995) Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci USA 92:5817–5821. https://doi.org/10.1073/pnas.92.13.5817
Tsakiridis T, Vranic M, Klip A (1995) Phosphatidylinositol 3-kinase and the actin network are not required for the stimulation of glucose transport caused by mitochondrial uncoupling: comparison with insulin action. Biochem J 309(Pt 1):1–5. https://doi.org/10.1042/bj3090001
Young LH, Coven DL, Russell RR 3rd (2000) Cellular and molecular regulation of cardiac glucose transport. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology 7:267–276. https://doi.org/10.1016/s1071-3581(00)70016-x
Zhou L, Huang H, Yuan CL, Keung W, Lopaschuk GD, Stanley WC (2008) Metabolic response to an acute jump in cardiac workload: effects on malonyl-CoA, mechanical efficiency, and fatty acid oxidation. Am J Physiol Heart Circ Physiol 294:H954-960. https://doi.org/10.1152/ajpheart.00557.2007
Beauloye C, Marsin AS, Bertrand L, Vanoverschelde JL, Rider MH, Hue L (2002) The stimulation of heart glycolysis by increased workload does not require AMP-activated protein kinase but a wortmannin-sensitive mechanism. FEBS Lett 531:324–328. https://doi.org/10.1016/s0014-5793(02)03552-4
Heidrich F, Schotola H, Popov AF, Sohns C, Schuenemann J, Friedrich M, Coskun KO, von Lewinski D, Hinz J, Bauer M, Mokashi SA, Sossalla S, Schmitto JD (2010) AMPK - Activated Protein Kinase and its Role in Energy Metabolism of the Heart. Curr Cardiol Rev 6:337–342. https://doi.org/10.2174/157340310793566073
Heidrich F, Sossalla S, Schotola H, Vorkamp T, Ortmann P, Popov AF, Coskun KO, Rajab TK, Friedrich M, Sohns C, Hinz J, Bauer M, Quintel M, Schondube FA, Schmitto JD (2010) The role of phospho-adenosine monophosphate-activated protein kinase and vascular endothelial growth factor in a model of chronic heart failure. Artif Organs 34:969–979. https://doi.org/10.1111/j.1525-1594.2010.01121.x
Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG (2006) Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation 114:1151–1158. https://doi.org/10.1161/CIRCULATIONAHA.106.613646
Doenst T, Nguyen TD, Abel ED (2013) Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113:709–724. https://doi.org/10.1161/CIRCRESAHA.113.300376
Velez M, Kohli S, Sabbah HN (2014) Animal models of insulin resistance and heart failure. Heart Fail Rev 19:1–13. https://doi.org/10.1007/s10741-013-9387-6
Bowman PRT, Smith GL, Gould GW (2019) Cardiac SNARE Expression in Health and Disease. Front Endocrinol 10:881. https://doi.org/10.3389/fendo.2019.00881
Liao R, Jain M, Cui L, D’Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R (2002) Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation 106:2125–2131. https://doi.org/10.1161/01.cir.0000034049.61181.f3
Pereira RO, Wende AR, Olsen C, Soto J, Rawlings T, Zhu Y, Anderson SM, Abel ED (2013) Inducible overexpression of GLUT1 prevents mitochondrial dysfunction and attenuates structural remodeling in pressure overload but does not prevent left ventricular dysfunction. J Am Heart Assoc 2:e000301. https://doi.org/10.1161/JAHA.113.000301
Pereira RO, Wende AR, Olsen C, Soto J, Rawlings T, Zhu Y, Riehle C, Abel ED (2014) GLUT1 deficiency in cardiomyocytes does not accelerate the transition from compensated hypertrophy to heart failure. J Mol Cell Cardiol 72:95–103. https://doi.org/10.1016/j.yjmcc.2014.02.011
Ma S, Meng Z, Chen R, Guan KL (2019) The Hippo pathway: biology and pathophysiology. Annu Rev Biochem 88:577–604. https://doi.org/10.1146/annurev-biochem-013118-111829
Gao C, Wang Y (2022) YAP: The nexus between metabolism and cardiac remodeling. J Clin Investig 132. https://doi.org/10.1172/JCI157664
Kashihara T, Mukai R, Oka SI, Zhai P, Nakada Y, Yang Z, Mizushima W, Nakahara T, Warren JS, Abdellatif M, Sadoshima J (2022) YAP mediates compensatory cardiac hypertrophy through aerobic glycolysis in response to pressure overload. J Clin Investig 132. https://doi.org/10.1172/JCI150595
Kaczmarczyk SJ, Andrikopoulos S, Favaloro J, Domenighetti AA, Dunn A, Ernst M, Grail D, Fodero-Tavoletti M, Huggins CE, Delbridge LM, Zajac JD, Proietto J (2003) Threshold effects of glucose transporter-4 (GLUT4) deficiency on cardiac glucose uptake and development of hypertrophy. J Mol Endocrinol 31:449–459. https://doi.org/10.1677/jme.0.0310449
Schilling JD, Mann DL (2012) Diabetic cardiomyopathy: bench to bedside. Heart Fail Clin 8:619–631. https://doi.org/10.1016/j.hfc.2012.06.007
Tsushima K, Bugger H, Wende AR, Soto J, Jenson GA, Tor AR, McGlauflin R, Kenny HC, Zhang Y, Souvenir R, Hu XX, Sloan CL, Pereira RO, Lira VA, Spitzer KW, Sharp TL, Shoghi KI, Sparagna GC, Rog-Zielinska EA, Kohl P, Khalimonchuk O, Schaffer JE, Abel ED (2018) Mitochondrial reactive oxygen species in lipotoxic hearts induce post-translational modifications of AKAP121, DRP1, and OPA1 that promote mitochondrial fission. Circ Res 122:58–73. https://doi.org/10.1161/CIRCRESAHA.117.311307
De Geest B, Mishra M (2022) Role of oxidative stress in diabetic cardiomyopathy. Antioxidants 11. https://doi.org/10.3390/antiox11040784
Arkadievich OD (2021) Metabolic markers of myocardium insulin resistance in dogs with heart failure. Open veterinary journal 10:363–370. https://doi.org/10.4314/ovj.v10i4.2
Wu R, Wyatt E, Chawla K, Tran M, Ghanefar M, Laakso M, Epting CL, Ardehali H (2012) Hexokinase II knockdown results in exaggerated cardiac hypertrophy via increased ROS production. EMBO Mol Med 4:633–646. https://doi.org/10.1002/emmm.201200240
Tsutsui H, Kinugawa S, Matsushima S (2011) Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301:H2181-2190. https://doi.org/10.1152/ajpheart.00554.2011
Ramachandra CJA, Cong S, Chan X, Yap EP, Yu F, Hausenloy DJ (2021) Oxidative stress in cardiac hypertrophy: from molecular mechanisms to novel therapeutic targets. Free Radical Biol Med 166:297–312. https://doi.org/10.1016/j.freeradbiomed.2021.02.040
McCommis KS, Douglas DL, Krenz M, Baines CP (2013) Cardiac-specific hexokinase 2 overexpression attenuates hypertrophy by increasing pentose phosphate pathway flux. J Am Heart Assoc 2. https://doi.org/10.1161/JAHA.113.000355
Badolia R, Ramadurai DKA, Abel ED, Ferrin P, Taleb I, Shankar TS, Krokidi AT, Navankasattusas S, McKellar SH, Yin M, Kfoury AG, Wever-Pinzon O, Fang JC, Selzman CH, Chaudhuri D, Rutter J, Drakos SG (2020) The role of nonglycolytic glucose metabolism in myocardial recovery upon mechanical unloading and circulatory support in chronic heart failure. Circulation 142:259–274. https://doi.org/10.1161/CIRCULATIONAHA.119.044452
Nakano H, Minami I, Braas D, Pappoe H, Wu X, Sagadevan A, Vergnes L, Fu K, Morselli M, Dunham C, Ding X, Stieg AZ, Gimzewski JK, Pellegrini M, Clark PM, Reue K, Lusis AJ, Ribalet B, Kurdistani SK, Christofk H, Nakatsuji N, Nakano A (2017) Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. eLife 6. https://doi.org/10.7554/eLife.29330
Hecker PA, Leopold JA, Gupte SA, Recchia FA, Stanley WC (2013) Impact of glucose-6-phosphate dehydrogenase deficiency on the pathophysiology of cardiovascular disease. Am J Physiol Heart Circ Physiol 304:H491-500. https://doi.org/10.1152/ajpheart.00721.2012
Jain M, Cui L, Brenner DA, Wang B, Handy DE, Leopold JA, Loscalzo J, Apstein CS, Liao R (2004) Increased myocardial dysfunction after ischemia-reperfusion in mice lacking glucose-6-phosphate dehydrogenase. Circulation 109:898–903. https://doi.org/10.1161/01.CIR.0000112605.43318.CA
Vimercati C, Qanud K, Mitacchione G, Sosnowska D, Ungvari Z, Sarnari R, Mania D, Patel N, Hintze TH, Gupte SA, Stanley WC, Recchia FA (2014) Beneficial effects of acute inhibition of the oxidative pentose phosphate pathway in the failing heart. Am J Physiol Heart Circ Physiol 306:H709-717. https://doi.org/10.1152/ajpheart.00783.2013
Gupte SA, Levine RJ, Gupte RS, Young ME, Lionetti V, Labinskyy V, Floyd BC, Ojaimi C, Bellomo M, Wolin MS, Recchia FA (2006) Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol 41:340–349. https://doi.org/10.1016/j.yjmcc.2006.05.003
Cairns M, Joseph D, Essop MF (2022) The dual role of the hexosamine biosynthetic pathway in cardiac physiology and pathophysiology. Front Endocrinol 13. https://doi.org/10.3389/fendo.2022.984342
Dassanayaka S, Jones SP (2014) O-GlcNAc and the cardiovascular system. Pharmacol Ther 142:62–71. https://doi.org/10.1016/j.pharmthera.2013.11.005
Collins HE, Chatham JC (2020) Regulation of cardiac O-GlcNAcylation: more than just nutrient availability. Biochim Biophys Acta Mol Basis Dis 1866:165712. https://doi.org/10.1016/j.bbadis.2020.165712
Mailleux F, Gelinas R, Beauloye C, Horman S, Bertrand L (2016) O-GlcNAcylation, enemy or ally during cardiac hypertrophy development? Biochem Biophys Acta 1862:2232–2243. https://doi.org/10.1016/j.bbadis.2016.08.012
Lunde IG, Aronsen JM, Kvaloy H, Qvigstad E, Sjaastad I, Tonnessen T, Christensen G, Gronning-Wang LM, Carlson CR (2012) Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol Genomics 44:162–172. https://doi.org/10.1152/physiolgenomics.00016.2011
Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan YT, Jones SP (2010) O-linked beta-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci USA 107:17797–17802. https://doi.org/10.1073/pnas.1001907107
Umapathi P, Mesubi OO, Banerjee PS, Abrol N, Wang Q, Luczak ED, Wu Y, Granger JM, Wei AC, Reyes Gaido OE, Florea L, Talbot CC Jr, Hart GW, Zachara NE, Anderson ME (2021) Excessive O-GlcNAcylation causes heart failure and sudden death. Circulation 143:1687–1703. https://doi.org/10.1161/CIRCULATIONAHA.120.051911
Liu Q, Chen Y, Auger-Messier M, Molkentin JD (2012) Interaction between NFkappaB and NFAT coordinates cardiac hypertrophy and pathological remodeling. Circ Res 110:1077–1086. https://doi.org/10.1161/CIRCRESAHA.111.260729
Dodge-Kafka K, Gildart M, Tokarski K, Kapiloff MS (2019) mAKAPbeta signalosomes - a nodal regulator of gene transcription associated with pathological cardiac remodeling. Cell Signal 63. https://doi.org/10.1016/j.cellsig.2019.109357
Han Y, Nie J, Wang DW, Ni L (2022) Mechanism of histone deacetylases in cardiac hypertrophy and its therapeutic inhibitors. Front Cardiovasc Med 9
Gelinas R, Mailleux F, Dontaine J, Bultot L, Demeulder B, Ginion A, Daskalopoulos EP, Esfahani H, Dubois-Deruy E, Lauzier B, Gauthier C, Olson AK, Bouchard B, Des RC, Viollet B, Sakamoto K, Balligand JL, Vanoverschelde JL, Beauloye C, Horman S, Bertrand L (2018) AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nat Commun 9:374. https://doi.org/10.1038/s41467-017-02795-4
Zhu WZ, El-Nachef D, Yang X, Ledee D, Olson AK (2019) O-GlcNAc transferase promotes compensated cardiac function and protein kinase A O-GlcNAcylation during early and established pathological hypertrophy from pressure overload. J Am Heart Assoc 8
Matsuno M, Yokoe S, Nagatsuka T, Morihara H, Moriwaki K, Asahi M (2023) O-GlcNAcylation-induced GSK-3beta activation deteriorates pressure overload-induced heart failure via lack of compensatory cardiac hypertrophy in mice. Front Endocrinol 14:1122125. https://doi.org/10.3389/fendo.2023.1122125
Dassanayaka S, Brainard RE, Watson LJ, Long BW, Brittian KR, DeMartino AM, Aird AL, Gumpert AM, Audam TN, Kilfoil PJ, Muthusamy S, Hamid T, Prabhu SD, Jones SP (2017) Cardiomyocyte Ogt limits ventricular dysfunction in mice following pressure overload without affecting hypertrophy. Basic Res Cardiol 112:23. https://doi.org/10.1007/s00395-017-0612-7
Wang C, Qiao S, Zhao Y, Tian H, Yan W, Hou X, Wang R, Zhang B, Yang C, Zhu F, Jiao Y, Jin J, Chen Y, Tian W (2023) The KLF7/PFKL/ACADL axis modulates cardiac metabolic remodelling during cardiac hypertrophy in male mice. Nat Commun 14:959. https://doi.org/10.1038/s41467-023-36712-9
Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, Tokarska-Schlattner M, Aasum E, Bogdanova A, Perriard E, Perriard JC, Larsen T, Pedrazzini T, Krek W (2009) Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab 9:512–524. https://doi.org/10.1016/j.cmet.2009.05.005
Ducker GS, Rabinowitz JD (2017) One-carbon metabolism in health and disease. Cell Metab 25:27–42. https://doi.org/10.1016/j.cmet.2016.08.009
Padron-Barthe L, Villalba-Orero M, Gomez-Salinero JM, Acin-Perez R, Cogliati S, Lopez-Olaneta M, Ortiz-Sanchez P, Bonzon-Kulichenko E, Vazquez J, Garcia-Pavia P, Rosenthal N, Enriquez JA, Lara-Pezzi E (2018) Activation of serine one-carbon metabolism by calcineurin abeta1 reduces myocardial hypertrophy and improves ventricular function. J Am Coll Cardiol 71:654–667. https://doi.org/10.1016/j.jacc.2017.11.067
Ma H, Yu S, Liu X, Zhang Y, Fakadej T, Liu Z, Yin C, Shen W, Locasale JW, Taylor JM, Qian L, Liu J (2019) Lin28a regulates pathological cardiac hypertrophic growth through Pck2-mediated enhancement of anabolic synthesis. Circulation 139:1725–1740. https://doi.org/10.1161/CIRCULATIONAHA.118.037803
Leong HS, Brownsey RW, Kulpa JE, Allard MF (2003) Glycolysis and pyruvate oxidation in cardiac hypertrophy–why so unbalanced? Comparative biochemistry and physiology. Part A, Molecular & integrative physiology 135:499–513. https://doi.org/10.1016/s1095-6433(03)00007-2
Lahey R, Carley AN, Wang X, Glass CE, Accola KD, Silvestry S, O’Donnell JM, Lewandowski ED (2018) Enhanced redox state and efficiency of glucose oxidation with miR based suppression of maladaptive NADPH-dependent malic enzyme 1 expression in hypertrophied hearts. Circ Res 122:836–845. https://doi.org/10.1161/CIRCRESAHA.118.312660
Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, Taegtmeyer H, O’Donnell JM, Lewandowski ED (2009) Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res 104:805–812. https://doi.org/10.1161/CIRCRESAHA.108.189951
Fillmore N, Levasseur JL, Fukushima A, Wagg CS, Wang W, Dyck JRB, Lopaschuk GD (2018) Uncoupling of glycolysis from glucose oxidation accompanies the development of heart failure with preserved ejection fraction. Mol Med 24:3. https://doi.org/10.1186/s10020-018-0005-x
Mori J, Basu R, McLean BA, Das SK, Zhang L, Patel VB, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY (2012) Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circ Heart Fail 5:493–503. https://doi.org/10.1161/CIRCHEARTFAILURE.112.966705
Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY (2013) ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am J Physiol Heart Circ Physiol 304:H1103-1113. https://doi.org/10.1152/ajpheart.00636.2012
Zhabyeyev P, Gandhi M, Mori J, Basu R, Kassiri Z, Clanachan A, Lopaschuk GD, Oudit GY (2013) Pressure-overload-induced heart failure induces a selective reduction in glucose oxidation at physiological afterload. Cardiovasc Res 97:676–685. https://doi.org/10.1093/cvr/cvs424
Lydell CP, Chan A, Wambolt RB, Sambandam N, Parsons H, Bondy GP, Rodrigues B, Popov KM, Harris RA, Brownsey RW, Allard MF (2002) Pyruvate dehydrogenase and the regulation of glucose oxidation in hypertrophied rat hearts. Cardiovasc Res 53:841–851. https://doi.org/10.1016/s0008-6363(01)00560-0
Gupte AA, Hamilton DJ, Cordero-Reyes AM, Youker KA, Yin Z, Estep JD, Stevens RD, Wenner B, Ilkayeva O, Loebe M, Peterson LE, Lyon CJ, Wong ST, Newgard CB, Torre-Amione G, Taegtmeyer H, Hsueh WA (2014) Mechanical unloading promotes myocardial energy recovery in human heart failure. Circ Cardiovasc Genet 7:266–276. https://doi.org/10.1161/CIRCGENETICS.113.000404
Cluntun AA, Badolia R, Lettlova S, Parnell KM, Shankar TS, Diakos NA, Olson KA, Taleb I, Tatum SM, Berg JA, Cunningham CN, Van Ry T, Bott AJ, Krokidi AT, Fogarty S, Skedros S, Swiatek WI, Yu X, Luo B, Merx S, Navankasattusas S, Cox JE, Ducker GS, Holland WL, McKellar SH, Rutter J, Drakos SG (2021) The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab 33(629–648)
Zhang X, Ji R, Liao X, Castillero E, Kennel PJ, Brunjes DL, Franz M, Mobius-Winkler S, Drosatos K, George I, Chen EI, Colombo PC, Schulze PC (2018) MicroRNA-195 regulates metabolism in failing myocardium via alterations in sirtuin 3 expression and mitochondrial protein acetylation. Circulation 137:2052–2067. https://doi.org/10.1161/CIRCULATIONAHA.117.030486
Abumrad NA, Cabodevilla AG, Samovski D, Pietka T, Basu D, Goldberg IJ (2021) Endothelial cell receptors in tissue lipid uptake and metabolism. Circ Res 128:433–450. https://doi.org/10.1161/CIRCRESAHA.120.318003
Samovski D, Jacome-Sosa M, Abumrad NA (2023) Fatty acid transport and signaling: mechanisms and physiological implications. Annu Rev Physiol 85:317–337. https://doi.org/10.1146/annurev-physiol-032122-030352
Luiken JJ, Coort SL, Willems J, Coumans WA, Bonen A, van der Vusse GJ, Glatz JF (2003) Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes 52:1627–1634. https://doi.org/10.2337/diabetes.52.7.1627
Glatz JFC, Luiken J (2018) Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J Lipid Res 59:1084–1093. https://doi.org/10.1194/jlr.R082933
Hsieh FL, Turner L, Bolla JR, Robinson CV, Lavstsen T, Higgins MK (2016) The structural basis for CD36 binding by the malaria parasite. Nat Commun 7:12837. https://doi.org/10.1038/ncomms12837
van der Meer DL, Degenhardt T, Vaisanen S, de Groot PJ, Heinaniemi M, de Vries SC, Muller M, Carlberg C, Kersten S (2010) Profiling of promoter occupancy by PPARalpha in human hepatoma cells via ChIP-chip analysis. Nucleic Acids Res 38:2839–2850. https://doi.org/10.1093/nar/gkq012
Shu H, Peng Y, Hang W, Nie J, Zhou N, Wang DW (2022) The role of CD36 in cardiovascular disease. Cardiovasc Res 118:115–129. https://doi.org/10.1093/cvr/cvaa319
Harmon CM, Luce P, Beth AH, Abumrad NA (1991) Labeling of adipocyte membranes by sulfo-N-succinimidyl derivatives of long-chain fatty acids: inhibition of fatty acid transport. J Membr Biol 121:261–268. https://doi.org/10.1007/BF01951559
Tanaka T, Nakata T, Oka T, Ogawa T, Okamoto F, Kusaka Y, Sohmiya K, Shimamoto K, Itakura K (2001) Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J Lipid Res 42:751–759
Oka S, Zhai P, Yamamoto T, Ikeda Y, Byun J, Hsu CP, Sadoshima J (2015) Peroxisome Proliferator activated receptor-alpha association with silent information regulator 1 suppresses cardiac fatty acid metabolism in the failing heart. Circ Heart Fail 8:1123–1132. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002216
Karam CN, Warren CM, Henze M, Banke NH, Lewandowski ED, Solaro RJ (2017) Peroxisome proliferator-activated receptor-alpha expression induces alterations in cardiac myofilaments in a pressure-overload model of hypertrophy. Am J Physiol Heart Circ Physiol 312:H681–H690. https://doi.org/10.1152/ajpheart.00469.2016
Ferrannini E, Baldi S, Scozzaro T, Tsimihodimos V, Tesfaye F, Shaw W, Rosenthal N, Figtree GA, Neal B, Mahaffey KW, Perkovic V, Hansen MK (2022) Fasting substrate concentrations predict cardiovascular outcomes in the CANagliflozin cardioVascular Assessment Study (CANVAS). Diabetes Care 45:1893–1899. https://doi.org/10.2337/dc21-2398
Goldenberg JR, Carley AN, Ji R, Zhang X, Fasano M, Schulze PC, Lewandowski ED (2019) Preservation of acyl coenzyme A attenuates pathological and metabolic cardiac remodeling through selective lipid trafficking. Circulation 139:2765–2777. https://doi.org/10.1161/CIRCULATIONAHA.119.039610
Weis BC, Cowan AT, Brown N, Foster DW, McGarry JD (1994) Use of a selective inhibitor of liver carnitine palmitoyltransferase I (CPT I) allows quantification of its contribution to total CPT I activity in rat heart. Evidence that the dominant cardiac CPT I isoform is identical to the skeletal muscle enzyme. J Biol Chem 269:26443–26448
Bartelds B, Takens J, Smid GB, Zammit VA, Prip-Buus C, Kuipers JR, van der Leij FR (2004) Myocardial carnitine palmitoyltransferase I expression and long-chain fatty acid oxidation in fetal and newborn lambs. Am J Physiol Heart Circ Physiol 286:H2243-2248. https://doi.org/10.1152/ajpheart.00864.2003
He L, Kim T, Long Q, Liu J, Wang P, Zhou Y, Ding Y, Prasain J, Wood PA, Yang Q (2012) Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation 126:1705–1716. https://doi.org/10.1161/CIRCULATIONAHA.111.075978
Lewandowski ED, Fischer SK, Fasano M, Banke NH, Walker LA, Huqi A, Wang X, Lopaschuk GD, O’Donnell JM (2013) Acute liver carnitine palmitoyltransferase I overexpression recapitulates reduced palmitate oxidation of cardiac hypertrophy. Circ Res 112:57–65. https://doi.org/10.1161/CIRCRESAHA.112.274456
Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ (2008) Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res 49:2101–2112. https://doi.org/10.1194/jlr.M800147-JLR200
Yazici D, Sezer H (2017) Insulin resistance, obesity and lipotoxicity. Adv Exp Med Biol 960:277–304. https://doi.org/10.1007/978-3-319-48382-5_12
Pereyra AS, Hasek LY, Harris KL, Berman AG, Damen FW, Goergen CJ, Ellis JM (2017) Loss of cardiac carnitine palmitoyltransferase 2 results in rapamycin-resistant, acetylation-independent hypertrophy. J Biol Chem 292:18443–18456. https://doi.org/10.1074/jbc.M117.800839
Verrey F (2003) System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch 445:529–533. https://doi.org/10.1007/s00424-002-0973-z
Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki H, Igarashi K, Dai Z, White PJ, McGarrah RW, Ilkayeva OR, Deleye Y, Oguri Y, Kuroda M, Ikeda K, Li H, Ueno A, Ohishi M, Ishikawa T, Kim K, Chen Y, Sponton CH, Pradhan RN, Majd H, Greiner VJ, Yoneshiro M, Brown Z, Chondronikola M, Takahashi H, Goto T, Kawada T, Sidossis L, Szoka FC, McManus MT, Saito M, Soga T, Kajimura S (2019) BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572:614–619. https://doi.org/10.1038/s41586-019-1503-x
Walejko JM, Christopher BA, Crown SB, Zhang GF, Pickar-Oliver A, Yoneshiro T, Foster MW, Page S, van Vliet S, Ilkayeva O, Muehlbauer MJ, Carson MW, Brozinick JT, Hammond CD, Gimeno RE, Moseley MA, Kajimura S, Gersbach CA, Newgard CB, White PJ, McGarrah RW (2021) Branched-chain alpha-ketoacids are preferentially reaminated and activate protein synthesis in the heart. Nat Commun 12:1680. https://doi.org/10.1038/s41467-021-21962-2
Biswas D, Duffley L, Pulinilkunnil T (2019) Role of branched-chain amino acid-catabolizing enzymes in intertissue signaling, metabolic remodeling, and energy homeostasis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 33:8711–8731. https://doi.org/10.1096/fj.201802842RR
White PJ, McGarrah RW, Grimsrud PA, Tso SC, Yang WH, Haldeman JM, Grenier-Larouche T, An J, Lapworth AL, Astapova I, Hannou SA, George T, Arlotto M, Olson LB, Lai M, Zhang GF, Ilkayeva O, Herman MA, Wynn RM, Chuang DT, Newgard CB (2018) The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab 27(1281–1293)
Zhou M, Lu G, Gao C, Wang Y, Sun H (2012) Tissue-specific and nutrient regulation of the branched-chain alpha-keto acid dehydrogenase phosphatase, protein phosphatase 2Cm (PP2Cm). J Biol Chem 287:23397–23406. https://doi.org/10.1074/jbc.M112.351031
Lu G, Sun H, She P, Youn JY, Warburton S, Ping P, Vondriska TM, Cai H, Lynch CJ, Wang Y (2009) Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Investig 119:1678–1687. https://doi.org/10.1172/JCI38151
Fillmore N, Wagg CS, Zhang L, Fukushima A, Lopaschuk GD (2018) Cardiac branched-chain amino acid oxidation is reduced during insulin resistance in the heart. Am J Physiol Endocrinol Metab 315:E1046–E1052. https://doi.org/10.1152/ajpendo.00097.2018
Uddin GM, Zhang L, Shah S, Fukushima A, Wagg CS, Gopal K, Al BR, Pherwani S, Ho KL, Boisvenue J, Karwi QG, Altamimi T, Wishart DS, Dyck JRB, Ussher JR, Oudit GY, Lopaschuk GD (2019) Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc Diabetol 18:86. https://doi.org/10.1186/s12933-019-0892-3
Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136:521–534. https://doi.org/10.1016/j.cell.2008.11.044
Lu G, Ren S, Korge P, Choi J, Dong Y, Weiss J, Koehler C, Chen JN, Wang Y (2007) A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev 21:784–796. https://doi.org/10.1101/gad.1499107
Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, Jeyaraj D, Youn JY, Ren S, Liu Y, Rau CD, Shah S, Ilkayeva O, Gui WJ, William NS, Wynn RM, Newgard CB, Cai H, Xiao X, Chuang DT, Schulze PC, Lynch C, Jain MK, Wang Y (2016) Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 133:2038–2049. https://doi.org/10.1161/CIRCULATIONAHA.115.020226
Li Z, Xia H, 3rd Sharp TE, LaPenna KB, Elrod JW, Casin KM, Liu K, Calvert JW, Chau VQ, Salloum FN, Xu S, Xian M, Nagahara N, Goodchild TT, Lefer DJ (2022) Mitochondrial H(2)S regulates BCAA catabolism in heart failure. Circ Res 131:222–235. https://doi.org/10.1161/CIRCRESAHA.121.319817
Yu JY, Cao N, Rau CD, Lee RP, Yang J, Flach RJR, Petersen L, Zhu C, Pak YL, Miller RA, Liu Y, Wang Y, Li Z, Sun H, Gao C (2023) Cell-autonomous effect of cardiomyocyte branched-chain amino acid catabolism in heart failure in mice. Acta Pharmacol Sin 44:1380–1390. https://doi.org/10.1038/s41401-023-01076-9
Puchalska P, Crawford PA (2017) Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 25:262–284. https://doi.org/10.1016/j.cmet.2016.12.022
Abdul Kadir A, Clarke K, Evans RD (2020) Cardiac ketone body metabolism. Biochim Biophys Acta Mol Basis Dis 1866
Hahn VS, Petucci C, Kim MS, Bedi KC Jr, Wang H, Mishra S, Koleini N, Yoo EJ, Margulies KB, Arany Z, Kelly DP, Kass DA, Sharma K (2023) Myocardial metabolomics of human heart failure with preserved ejection fraction. Circulation 147:1147–1161. https://doi.org/10.1161/CIRCULATIONAHA.122.061846
Flores-Guerrero JL, Westenbrink BD, Connelly MA, Otvos JD, Groothof D, Shalaurova I, Garcia E, Navis G, de Boer RA, Bakker SJL, Dullaart RPF (2021) Association of beta-hydroxybutyrate with development of heart failure: Sex differences in a Dutch population cohort. Eur J Clin Investig 51
Ho KL, Zhang L, Wagg C, Al BR, Gopal K, Levasseur J, Leone T, Dyck JRB, Ussher JR, Muoio DM, Kelly DP, Lopaschuk GD (2019) Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res 115:1606–1616. https://doi.org/10.1093/cvr/cvz045
Parreira RC, Gomez-Mendoza DP, de Jesus ICG, Lemos RP, Santos AK, Rezende CP, Figueiredo HCP, Pinto MCX, Kjeldsen F, Guatimosim S, Resende RR, Verano-Braga T (2020) Cardiomyocyte proteome remodeling due to isoproterenol-induced cardiac hypertrophy during the compensated phase. Proteom Clin Appl 14
Uchihashi M, Hoshino A, Okawa Y, Ariyoshi M, Kaimoto S, Tateishi S, Ono K, Yamanaka R, Hato D, Fushimura Y, Honda S, Fukai K, Higuchi Y, Ogata T, Iwai-Kanai E, Matoba S (2017) Cardiac-specific Bdh1 overexpression ameliorates oxidative stress and cardiac remodeling in pressure overload-induced heart failure. Circ Heart Fail 10. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004417
Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, Recchia FA, Kelly DP (2019) The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 4. https://doi.org/10.1172/jci.insight.124079
Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE (2016) Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 133:706–716. https://doi.org/10.1161/CIRCULATIONAHA.115.017545
Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Kruger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP (2016) The failing heart relies on ketone bodies as a fuel. Circulation 133:698–705. https://doi.org/10.1161/CIRCULATIONAHA.115.017355
Byrne NJ, Soni S, Takahara S, Ferdaoussi M, Al Batran R, Darwesh AM, Levasseur JL, Beker D, Vos DY, Schmidt MA, Alam AS, Maayah ZH, Schertzer JD, Seubert JM, Ussher JR, Dyck JRB (2020) Chronically elevating circulating ketones can reduce cardiac inflammation and blunt the development of heart failure. Circ Heart Fail 13
Schugar RC, Moll AR, Andre DD, Weinheimer CJ, Kovacs A, Crawford PA (2014) Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Molecular metabolism 3:754–769. https://doi.org/10.1016/j.molmet.2014.07.010
Possik E, Madiraju SRM, Prentki M (2017) Glycerol-3-phosphate phosphatase/PGP: Role in intermediary metabolism and target for cardiometabolic diseases. Biochimie 143:18–28. https://doi.org/10.1016/j.biochi.2017.08.001
Nakamura MT, Yudell BE, Loor JJ (2014) Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res 53:124–144. https://doi.org/10.1016/j.plipres.2013.12.001
Zara V, Assalve G, Ferramosca A (2022) Multiple roles played by the mitochondrial citrate carrier in cellular metabolism and physiology. Cellular and molecular life sciences : CMLS 79:428. https://doi.org/10.1007/s00018-022-04466-0
Wang Y, Yu W, Li S, Guo D, He J, Wang Y (2022) Acetyl-CoA Carboxylases and Diseases. Front Oncol 12:836058
Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJP, Thompson PD, Libby P, Cho L, Plutzky J, Bays HE, Moriarty PM, Menon V, Grobbee DE, Louie MJ, Chen CF, Li N, Bloedon L, Robinson P, Horner M, Sasiela WJ, McCluskey J, Davey D, Fajardo-Campos P, Petrovic P, Fedacko J, Zmuda W, Lukyanov Y, Nicholls SJ, Investigators CO (2023) Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med 388:1353–1364. https://doi.org/10.1056/NEJMoa2215024
Nissen SE, Menon V, Nicholls SJ, Brennan D, Laffin L, Ridker P, Ray KK, Mason D, Kastelein JJP, Cho L, Libby P, Li N, Foody J, Louie MJ, Lincoff AM (2023) Bempedoic acid for primary prevention of cardiovascular events in statin-intolerant patients. JAMA 330:131–140. https://doi.org/10.1001/jama.2023.9696
Mallipattu SK, Liu R, Zheng F, Narla G, Ma’ayan A, Dikman S, Jain MK, Saleem M, D’Agati V, Klotman P, Chuang PY, He JC (2012) Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation. J Biol Chem 287:19122–19135. https://doi.org/10.1074/jbc.M112.345983
Fan L, Hsieh PN, Sweet DR, Jain MK (2018) Kruppel-like factor 15: Regulator of BCAA metabolism and circadian protein rhythmicity. Pharmacol Res 130:123–126. https://doi.org/10.1016/j.phrs.2017.12.018
Shao D, Villet O, Zhang Z, Choi SW, Yan J, Ritterhoff J, Gu H, Djukovic D, Christodoulou D, Kolwicz SC Jr, Raftery D, Tian R (2018) Glucose promotes cell growth by suppressing branched-chain amino acid degradation. Nat Commun 9:2935. https://doi.org/10.1038/s41467-018-05362-7
Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR (2000) Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130:2413–2419. https://doi.org/10.1093/jn/130.10.2413
Karwi QG, Lopaschuk GD (2023) Branched-chain amino acid metabolism in the failing heart. Cardiovasc Drugs Ther 37:413–420. https://doi.org/10.1007/s10557-022-07320-4
Dimou A, Tsimihodimos V, Bairaktari E (2022) The critical role of the branched chain amino acids (BCAAs) catabolism-regulating enzymes, branched-chain aminotransferase (BCAT) and branched-chain alpha-keto acid dehydrogenase (BCKD), in human pathophysiology. Int J Mol Sci 23. https://doi.org/10.3390/ijms23074022
Li T, Zhang Z, Kolwicz SC Jr, Abell L, Roe ND, Kim M, Zhou B, Cao Y, Ritterhoff J, Gu H, Raftery D, Sun H, Tian R (2017) Defective Branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab 25:374–385. https://doi.org/10.1016/j.cmet.2016.11.005
Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, Baca LG, Kim E, Ghosh CC, Parikh SM, Jiang A, Chu Q, Forman DE, Lecker SH, Krishnaiah S, Rabinowitz JD, Weljie AM, Baur JA, Kasper DL, Arany Z (2016) A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med 22:421–426. https://doi.org/10.1038/nm.4057
Korf-Klingebiel M, Reboll MR, Polten F, Weber N, Jackle F, Wu X, Kallikourdis M, Kunderfranco P, Condorelli G, Giannitsis E, Kustikova OS, Schambach A, Pich A, Widder JD, Bauersachs J, van den Heuvel J, Kraft T, Wang Y, Wollert KC (2021) Myeloid-derived growth factor protects against pressure overload-induced heart failure by preserving sarco/endoplasmic reticulum Ca(2+)-ATPase expression in cardiomyocytes. Circulation 144:1227–1240. https://doi.org/10.1161/CIRCULATIONAHA.120.053365
Stromer H, Palmieri EA, De Groot MC, Di Rella F, Leupold A, Horn M, Monti MG, Napoli R, Di Gianni A, Isgaard J, Sacca L, Neubauer S, Cittadini A (2006) Growth hormone- and pressure overload-induced cardiac hypertrophy evoke different responses to ischemia-reperfusion and mechanical stretch. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society 16:29–40. https://doi.org/10.1016/j.ghir.2005.09.002
Mozaffari MS, Patel C, Schaffer SW (2006) Mechanisms underlying afterload-induced exacerbation of myocardial infarct size: role of T-type Ca2+ channel. Hypertension 47:912–919. https://doi.org/10.1161/01.HYP.0000209940.65941.46
Xu X, Zhen PH, Yu FC, Wang T, Li SN, Wei Q, Tong JY (2022) Chronic intermittent hypoxia accelerates cardiac dysfunction and cardiac remodeling during cardiac pressure overload in mice and can be alleviated by PHD3 overexpression. Front Cardiovasc Med 9
Ma LL, Kong FJ, Dong Z, Xin KY, Wang XX, Sun AJ, Zou YZ, Ge JB (2021) Hypertrophic preconditioning attenuates myocardial ischaemia-reperfusion injury by modulating SIRT3-SOD2-mROS-dependent autophagy. Cell Prolif 54
Ma LL, Ding ZW, Yin PP, Wu J, Hu K, Sun AJ, Zou YZ, Ge JB (2021) Hypertrophic preconditioning cardioprotection after myocardial ischaemia/reperfusion injury involves ALDH2-dependent metabolism modulation. Redox Biol 43
Ma L, Shi H, Li Y, Gao W, Guo J, Zhu J, Dong Z, Sun A, Zou Y, Ge J (2021) Hypertrophic preconditioning attenuates myocardial ischemia/reperfusion injury through the deacetylation of isocitrate dehydrogenase 2. Sci Bull 66:2099–2114. https://doi.org/10.1016/j.scib.2021.04.008
Correale M, Tricarico L, Croella F, Alfieri S, Fioretti F, Brunetti ND, Inciardi RM, Nodari S (2023) Novelties in the pharmacological approaches for chronic heart failure: new drugs and cardiovascular targets. Front Cardiovasc Med 10:1157472. https://doi.org/10.3389/fcvm.2023.1157472
Bersin RM, Wolfe C, Kwasman M, Lau D, Klinski C, Tanaka K, Khorrami P, Henderson GN, de Marco T, Chatterjee K (1994) Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate. J Am Coll Cardiol 23:1617–1624. https://doi.org/10.1016/0735-1097(94)90665-3
Wargovich TJ, MacDonald RG, Hill JA, Feldman RL, Stacpoole PW, Pepine CJ (1988) Myocardial metabolic and hemodynamic effects of dichloroacetate in coronary artery disease. Am J Cardiol 61:65–70. https://doi.org/10.1016/0002-9149(88)91306-9
Wilson JR, Mancini DM, Ferraro N, Egler J (1988) Effect of dichloroacetate on the exercise performance of patients with heart failure. J Am Coll Cardiol 12:1464–1469. https://doi.org/10.1016/s0735-1097(88)80010-x
Abdelmalak M, Lew A, Ramezani R, Shroads AL, Coats BS, Langaee T, Shankar MN, Neiberger RE, Subramony SH, Stacpoole PW (2013) Long-term safety of dichloroacetate in congenital lactic acidosis. Mol Genet Metab 109:139–143. https://doi.org/10.1016/j.ymgme.2013.03.019
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsboll T (2016) Investigators S.-. Semaglutide and Cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375:1834–1844. https://doi.org/10.1056/NEJMoa1607141
Lingvay I., Brown-Frandsen K., Colhoun H. M., Deanfield J., Emerson S. S., Esbjerg S., Hardt-Lindberg S., Hovingh G. K., Kahn S. E., Kushner R. F., Lincoff A. M., Marso S. P., Fries T. M., Plutzky J., Ryan D. H., Group S. S. (2023) Semaglutide for cardiovascular event reduction in people with overweight or obesity: SELECT study baseline characteristics. Obesity 31:111–122. https://doi.org/10.1002/oby.23621
McGuire DK, Busui RP, Deanfield J, Inzucchi SE, Mann JFE, Marx N, Mulvagh SL, Poulter N, Engelmann MDM, Hovingh GK, Ripa MS, Gislum M, Brown-Frandsen K, Buse JB (2023) Effects of oral semaglutide on cardiovascular outcomes in individuals with type 2 diabetes and established atherosclerotic cardiovascular disease and/or chronic kidney disease: Design and baseline characteristics of SOUL, a randomized trial. Diabetes Obes Metab 25:1932–1941. https://doi.org/10.1111/dom.15058
Kosiborod MN, Abildstrom SZ, Borlaug BA, Butler J, Christensen L, Davies M, Hovingh KG, Kitzman DW, Lindegaard ML, Moller DV, Shah SJ, Treppendahl MB, Verma S, Petrie MC (2023) Design and baseline characteristics of STEP-HFpEF program evaluating semaglutide in patients with obesity HFpEF phenotype. JACC Heart failure 11:1000–1010. https://doi.org/10.1016/j.jchf.2023.05.010
Schmidt-Schweda S, Holubarsch C (2000) First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci 99:27–35
Holubarsch CJ, Rohrbach M, Karrasch M, Boehm E, Polonski L, Ponikowski P, Rhein S (2007) A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci 113:205–212. https://doi.org/10.1042/CS20060307
Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, Ashrafian H, Horowitz J, Fraser AG, Clarke K, Frenneaux M (2005) Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation 112:3280–3288. https://doi.org/10.1161/CIRCULATIONAHA.105.551457
Lee L, Horowitz J, Frenneaux M (2004) Metabolic manipulation in ischaemic heart disease, a novel approach to treatment. Eur Heart J 25:634–641. https://doi.org/10.1016/j.ehj.2004.02.018
Qian G, Jiang X, Jiang Z, Li T, Dong W, Guo J, Chen Y (2023) Early Trimetazidine therapy in patients undergoing primary percutaneous coronary intervention for ST segment elevation myocardial infarction reduces myocardial infarction size. Cardiovasc Drugs Ther 37:497–506. https://doi.org/10.1007/s10557-021-07259-y
Dai ZL, Song YF, Tian Y, Li Y, Lin M, Lin J, Wang Q, Wang P, Gao WL (2021) Trimetazidine offers myocardial protection in elderly coronary artery disease patients undergoing non-cardiac surgery: a randomized, double-blind, placebo-controlled trial. BMC Cardiovasc Disord 21:473. https://doi.org/10.1186/s12872-021-02287-w
Bohdan M, Stopczynska I, Wisniewski P, Morys J, Niedoszytko P, Gruchala M (2022) Effects of trimetazidine in patients with severe chronic heart failure with reduced left ventricular ejection fraction: a prospective, randomized, open-label, cross-over study. Cardiol J 29:627–636. https://doi.org/10.5603/CJ.a2020.0165
Harjoko RP, Sobirin MA, Uddin I, Bahrudin U, Maharani N, Herminingsih S, Tsutsui H (2022) Trimetazidine improves left ventricular global longitudinal strain value in patients with heart failure with reduced ejection fraction due to ischemic heart disease. Drug Discov Ther 16:177–184. https://doi.org/10.5582/ddt.2022.01020
Nielsen R, Moller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frokiaer J, Eiskjaer H, Jespersen NR, Mellemkjaer S, Lassen TR, Pryds K, Botker HE, Wiggers H (2019) Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation 139:2129–2141. https://doi.org/10.1161/CIRCULATIONAHA.118.036459
Monzo L, Sedlacek K, Hromanikova K, Tomanova L, Borlaug BA, Jabor A, Kautzner J, Melenovsky V (2021) Myocardial ketone body utilization in patients with heart failure: the impact of oral ketone ester. Metab Clin Exp 115
Berg-Hansen K, Christensen KH, Gopalasingam N, Nielsen R, Eiskjaer H, Moller N, Birkelund T, Christensen S, Wiggers H (2023) Beneficial effects of ketone ester in patients with cardiogenic shock: a randomized, controlled, double-blind trial. JACC Heart Fail. https://doi.org/10.1016/j.jchf.2023.05.029
McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM, Committees D-HT (2019) Investigators, Dapagliflozin in Patients with heart failure and reduced ejection fraction. N Engl J Med 381:1995–2008. https://doi.org/10.1056/NEJMoa1911303
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Pina IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M, Investigators EM-PT (2021) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385:1451–1461. https://doi.org/10.1056/NEJMoa2107038
Butler J, Usman MS, Filippatos G, Ferreira JP, Bohm M, Brueckmann M, Januzzi JL, Kaul S, Pina IL, Ponikowski P, Senni M, Sumin M, Verma S, Zaremba-Pechmann L, Pocock SJ, Packer M, Anker S (2023) Safety and efficacy of empagliflozin and diuretic use in patients with heart failure and preserved ejection fraction: a post hoc analysis of the EMPEROR-preserved Trial. JAMA Cardiol 8:640–649. https://doi.org/10.1001/jamacardio.2023.1090
Tromp J, Ouwerkerk W, van Veldhuisen DJ, Hillege HL, Richards AM, van der Meer P, Anand IS, Lam CSP, Voors AA (2022) A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail 10:73–84. https://doi.org/10.1016/j.jchf.2021.09.004
Saucedo-Orozco H, Voorrips SN, Yurista SR, de Boer RA, Westenbrink BD (2022) SGLT2 inhibitors and ketone metabolism in heart failure. J Lipid Atheroscler 11:1–19. https://doi.org/10.12997/jla.2022.11.1.1
Hundertmark MJ, Adler A, Antoniades C, Coleman R, Griffin JL, Holman RR, Lamlum H, Lee J, Massey D, Miller J, Milton JE, Monga S, Mozes FE, Nazeer A, Raman B, Rider O, Rodgers CT, Valkovic L, Wicks E, Mahmod M, Neubauer S (2023) Assessment of cardiac energy metabolism, function, and physiology in patients with heart failure taking empagliflozin: the randomized, controlled EMPA-VISION trial. Circulation 147:1654–1669. https://doi.org/10.1161/CIRCULATIONAHA.122.062021
Selvaraj S, Fu Z, Jones P, Kwee LC, Windsor SL, Ilkayeva O, Newgard CB, Margulies KB, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Lanfear DE, Nassif ME, Javaheri A, Mentz RJ, Kosiborod MN, Shah SH, Investigators D-H (2022) Metabolomic profiling of the effects of dapagliflozin in heart failure with reduced ejection fraction: DEFINE-HF. Circulation 146:808–818. https://doi.org/10.1161/CIRCULATIONAHA.122.060402
Ferrannini E, Mark M, Mayoux E (2016) CV Protection in the EMPA-REG OUTCOME Trial: a “thrifty substrate” hypothesis. Diabetes Care 39:1108–1114. https://doi.org/10.2337/dc16-0330
Funding
This work was supported by the Tibet Natural Science Foundation of China (Grant No. XZ2020ZR-ZY55(z)), the National Natural Science Foundation of China (Grant No. 82160157), the National Natural Science Foundation of China (Grant No. 82002095), and the Funding by Science and Technology Projects in Guangzhou (Grant No. 202201011129).
Author information
Authors and Affiliations
Contributions
J. W. and X. D. helped search the literature and prepare the manuscript. J. C., D. Z., and J. X. helped with the article modification. J. Z. and S. W. helped prepare the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, J., Duan, X., Chen, J. et al. Metabolic adaptations in pressure overload hypertrophic heart. Heart Fail Rev 29, 95–111 (2024). https://doi.org/10.1007/s10741-023-10353-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-023-10353-y