Abstract
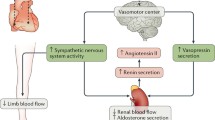
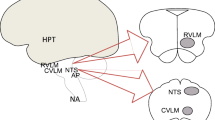
The pathophysiology of heart failure (HF) is characterized by an abnormal activation of neurohumoral systems, including the sympathetic nervous and the renin–angiotensin–aldosterone systems, which have long-term deleterious effects on the disease progression. Perpetuation of this neurohumoral activation is partially dependent of central nervous system (CNS) pathways, mainly involving the paraventricular nucleus of the hypothalamus and some regions of the brainstem. Modifications in these integrative CNS circuits result in the attenuation of sympathoinhibitory and exacerbation of sympathoexcitatory pathways. In addition to the regulation of sympathetic outflow, these central pathways coordinate a complex network of agents with an established pathophysiological relevance in HF such as angiotensin, aldosterone, and proinflammatory cytokines. Central pathways could be potential targets in HF therapy since the current mainstay of HF pharmacotherapy aims primarily at antagonizing the peripheral mechanisms. Thus, in the present review, we describe the role of CNS pathways in HF pathophysiology and as potential novel therapeutic targets.







Similar content being viewed by others
References
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P (2012) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 14(8):803–869. doi:10.1093/eurjhf/hfs105
Parati G, Esler M (2012) The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J 33(9):1058–1066. doi:10.1093/eurheartj/ehs041
Unger T, Li J (2004) The role of the renin–angiotensin–aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst 5(Suppl 1):S7–S10. doi:10.3317/jraas.2004.024
Hedayat M, Mahmoudi MJ, Rose NR, Rezaei N (2010) Proinflammatory cytokines in heart failure: double-edged swords. Heart Fail Rev 15(6):543–562. doi:10.1007/s10741-010-9168-4
Xu-Cai YO, Wu Q (2010) Molecular forms of natriuretic peptides in heart failure and their implications. Heart 96(6):419–424. doi:10.1136/hrt.2008.164145
Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP (2004) The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol 84(2–3):217–232. doi:10.1016/j.pbiomolbio.2003.11.010
Grassi G, Seravalle G, Quarti-Trevano F, Dell’oro R (2009) Sympathetic activation in congestive heart failure: evidence, consequences and therapeutic implications. Curr Vasc Pharmacol 7(2):137–145
Esler M, Kaye D, Lambert G, Esler D, Jennings G (1997) Adrenergic nervous system in heart failure. Am J Cardiol 80(11A):7L–14L
Ji XF, Shuo W, Yang L, Li CS (2012) Impaired beta-adrenergic receptor signalling in post-resuscitation myocardial dysfunction. Resuscitation 83(5):640–644. doi:10.1016/j.resuscitation.2011.11.014
Dorn GW 2nd (2002) Adrenergic pathways and left ventricular remodeling. J Card Fail 8(6 Suppl):S370–S373. doi:10.1054/jcaf.2002.129267
Weiss ML, Kenney MJ, Musch TI, Patel KP (2003) Modifications to central neural circuitry during heart failure. Acta Physiol Scand 177(1):57–67
Zucker IH, Patel KP, Schultz HD (2012) Neurohumoral stimulation. Heart Fail Clin 8(1):87–99. doi:10.1016/j.hfc.2011.08.007
Francis J, Wei SG, Weiss RM, Felder RB (2004) Brain angiotensin-converting enzyme activity and autonomic regulation in heart failure. Am J Physiol Heart Circ Physiol 287(5):H2138–H2146. doi:10.1152/ajpheart.00112.2004
Felder RB, Yu Y, Zhang ZH, Wei SG (2009) Pharmacological treatment for heart failure: a view from the brain. Clin Pharmacol Ther 86(2):216–220. doi:10.1038/clpt.2009.117
Tamargo J, Lopez-Sendon J (2011) Novel therapeutic targets for the treatment of heart failure. Nat Rev Drug Discov 10(7):536–555. doi:10.1038/nrd3431
Rickard J, Cheng A, Spragg D, Cantillon D, Baranowski B, Varma N, Wilkoff BL, Tang WW (2013) A clinical prediction rule to identify patients at heightened risk for early demise following cardiac resynchronization therapy. J Cardiovasc Electrophysiol. doi:10.1111/jce.12344
Singh JP, Gras D (2012) Biventricular pacing: current trends and future strategies. Eur Heart J 33(3):305–313. doi:10.1093/eurheartj/ehr366
Xu J, Hering D, Sata Y, Walton A, Krum H, Esler MD, Schlaich MP (2014) Renal denervation: current implications and future perspectives. Clin Sci (Lond) 126(1):41–53. doi:10.1042/CS20120581
Hauptman PJ, Schwartz PJ, Gold MR, Borggrefe M, Van Veldhuisen DJ, Starling RC, Mann DL (2012) Rationale and study design of the increase of vagal tone in heart failure study: INOVATE-HF. Am Heart J 163(6):954–962 e951. doi:10.1016/j.ahj.2012.03.021
Du YH, Chen AF (2007) A “love triangle” elicited by electrochemistry: complex interactions among cardiac sympathetic afferent, chemo-, and baroreflexes. J Appl Physiol 102(1):9–10. doi:10.1152/japplphysiol.01032.2006
Schultz HD (2001) Cardiac vagal chemosensory afferents. Function in pathophysiological states. Ann N Y Acad Sci 940:59–73
Guyenet PG (2006) The sympathetic control of blood pressure. Nat Rev Neurosci 7(5):335–346. doi:10.1038/nrn1902
Thames MD (1978) Contribution of cardiopulmonary baroreceptors to the control of the kidney. Fed Proc 37(5):1209–1213
Bishop VS, Thames MD, Schmid PG (1984) Effects of bilateral vagal cold block on vasopressin in conscious dogs. Am J Physiol 246(4 Pt 2):R566–R569
Brandle M, Wang W, Zucker IH (1994) Ventricular mechanoreflex and chemoreflex alterations in chronic heart failure. Circ Res 74(2):262–270
Gao L, Pan YX, Wang WZ, Li YL, Schultz HD, Zucker IH, Wang W (2007) Cardiac sympathetic afferent stimulation augments the arterial chemoreceptor reflex in anesthetized rats. J Appl Physiol 102(1):37–43. doi:10.1152/japplphysiol.00681.2006
Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W (2008) Interaction between cardiac sympathetic afferent reflex and chemoreflex is mediated by the NTS AT1 receptors in heart failure. Am J Physiol Heart Circ Physiol 295(3):H1216–H1226. doi:10.1152/ajpheart.00557.2008
Gan XB, Duan YC, Xiong XQ, Li P, Cui BP, Gao XY, Zhu GQ (2011) Inhibition of cardiac sympathetic afferent reflex and sympathetic activity by baroreceptor and vagal afferent inputs in chronic heart failure. PLoS ONE 6(10):e25784. doi:10.1371/journal.pone.0025784
Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, De W, Zhu GQ (2011) Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf) 203(2):289–297. doi:10.1111/j.1748-1716.2011.02313.x
Wang W, Ma R (2000) Cardiac sympathetic afferent reflexes in heart failure. Heart Fail Rev 5(1):57–71. doi:10.1023/A:1009898107964
Li YL, Xia XH, Zheng H, Gao L, Li YF, Liu D, Patel KP, Wang W, Schultz HD (2006) Angiotensin II enhances carotid body chemoreflex control of sympathetic outflow in chronic heart failure rabbits. Cardiovasc Res 71(1):129–138. doi:10.1016/j.cardiores.2006.03.017
Li YL, Li YF, Liu D, Cornish KG, Patel KP, Zucker IH, Channon KM, Schultz HD (2005) Gene transfer of neuronal nitric oxide synthase to carotid body reverses enhanced chemoreceptor function in heart failure rabbits. Circ Res 97(3):260–267. doi:10.1161/01.RES.0000175722.21555.55
Wang Y, Patel KP, Cornish KG, Channon KM, Zucker IH (2003) nNOS gene transfer to RVLM improves baroreflex function in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 285(4):H1660–H1667. doi:10.1152/ajpheart.00239.2003
Wang W, McClain JM, Zucker IH (1992) Aldosterone reduces baroreceptor discharge in the dog. Hypertension 19(3):270–277
Dibner-Dunlap ME, Thames MD (1992) Control of sympathetic nerve activity by vagal mechanoreflexes is blunted in heart failure. Circulation 86(6):1929–1934
Zucker IH, Share L, Gilmore JP (1979) Renal effects of left atrial distension in dogs with chronic congestive heart failure. Am J Physiol 236(4):H554–H560
Coote JH (2005) A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol 90(2):169–173. doi:10.1113/expphysiol.2004.029041
Wang WZ, Gao L, Pan YX, Zucker IH, Wang W (2007) AT1 receptors in the nucleus tractus solitarii mediate the interaction between the baroreflex and the cardiac sympathetic afferent reflex in anesthetized rats. Am J Physiol Regul Integr Comp Physiol 292(3):R1137–R1145. doi:10.1152/ajpregu.00590.2006
Ponikowski P, Chua TP, Piepoli M, Ondusova D, Webb-Peploe K, Harrington D, Anker SD, Volterrani M, Colombo R, Mazzuero G, Giordano A, Coats AJ (1997) Augmented peripheral chemosensitivity as a potential input to baroreflex impairment and autonomic imbalance in chronic heart failure. Circulation 96(8):2586–2594
Despas F, Lambert E, Vaccaro A, Labrunee M, Franchitto N, Lebrin M, Galinier M, Senard JM, Lambert G, Esler M, Pathak A (2012) Peripheral chemoreflex activation contributes to sympathetic baroreflex impairment in chronic heart failure. J Hypertens 30(4):753–760. doi:10.1097/HJH.0b013e328350136c
Gao L, Schultz HD, Patel KP, Zucker IH, Wang W (2005) Augmented input from cardiac sympathetic afferents inhibits baroreflex in rats with heart failure. Hypertension 45(6):1173–1181. doi:10.1161/01.HYP.0000168056.66981.c2
Sawchenko PE, Swanson LW (1982) The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 257(3):275–325
Affleck VS, Coote JH, Pyner S (2012) The projection and synaptic organisation of NTS afferent connections with presympathetic neurons, GABA and nNOS neurons in the paraventricular nucleus of the hypothalamus. Neuroscience 219:48–61. doi:10.1016/j.neuroscience.2012.05.070
Li YF, Patel KP (2003) Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand 177(1):17–26
Badoer E (2001) Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol 28(1–2):95–99
Ericsson A, Kovacs KJ, Sawchenko PE (1994) A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci 14(2):897–913
Watkins ND, Cork SC, Pyner S (2009) An immunohistochemical investigation of the relationship between neuronal nitric oxide synthase, GABA and presympathetic paraventricular neurons in the hypothalamus. Neuroscience 159(3):1079–1088. doi:10.1016/j.neuroscience.2009.01.012
Shafton AD, Ryan A, Badoer E (1998) Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801(1–2):239–243
Pyner S, Coote JH (2000) Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 100(3):549–556
Geerling JC, Shin JW, Chimenti PC, Loewy AD (2010) Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol 518(9):1460–1499. doi:10.1002/cne.22283
Polson JW, Mrljak S, Potts PD, Dampney RA (2002) Fos expression in spinally projecting neurons after hypotension in the conscious rabbit. Auton Neurosci 100(1–2):10–20
Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW (2003) Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand 177(3):209–218. doi:10.1046/j.1365-201X.2003.01070.x
Schreihofer AM, Guyenet PG (2002) The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29(5–6):514–521
Kenney MJ, Weiss ML, Haywood JR (2003) The paraventricular nucleus: an important component of the central neurocircuitry regulating sympathetic nerve outflow. Acta Physiol Scand 177(1):7–15
Li YF, Mayhan WG, Patel KP (2001) NMDA-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Heart Circ Physiol 281(6):H2328–H2336
Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP (2006) Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol 291(6):H2847–H2856. doi:10.1152/ajpheart.00625.2005
Zheng H, Liu X, Li Y, Sharma NM, Patel KP (2011) Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension 58(5):966–973. doi:10.1161/HYPERTENSIONAHA.111.176222
Sharma NM, Zheng H, Li YF, Patel KP (2012) Nitric oxide inhibits the expression of AT1 receptors in neurons. Am J Physiol Cell Physiol 302(8):C1162–C1173. doi:10.1152/ajpcell.00258.2011
Zhang K, Li YF, Patel KP (2001) Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol 281(3):H995–H1004
Felder RB, Francis J, Zhang ZH, Wei SG, Weiss RM, Johnson AK (2003) Heart failure and the brain: new perspectives. Am J Physiol Regul Integr Comp Physiol 284(2):R259–R276. doi:10.1152/ajpregu.00317.2002
Li DP, Chen SR, Finnegan TF, Pan HL (2004) Signalling pathway of nitric oxide in synaptic GABA release in the rat paraventricular nucleus. J Physiol 554(Pt 1):100–110. doi:10.1113/jphysiol.2003.053371
Contestabile A (2008) Regulation of transcription factors by nitric oxide in neurons and in neural-derived tumor cells. Prog Neurobiol 84(4):317–328. doi:10.1016/j.pneurobio.2008.01.002
Stern JE (2004) Nitric oxide and homeostatic control: an intercellular signalling molecule contributing to autonomic and neuroendocrine integration? Prog Biophys Mol Biol 84(2–3):197–215. doi:10.1016/j.pbiomolbio.2003.11.015
Kadekaro M, Liu H, Terrell ML, Gestl S, Bui V, Summy-Long JY (1997) Role of NO on vasopressin and oxytocin release and blood pressure responses during osmotic stimulation in rats. Am J Physiol 273(3 Pt 2):R1024–R1030
Martins-Pinge MC, Mueller PJ, Foley CM, Heesch CM, Hasser EM (2012) Regulation of arterial pressure by the paraventricular nucleus in conscious rats: interactions among glutamate, GABA, and nitric oxide. Front Physiol 3:490. doi:10.3389/fphys.2012.00490
Li YF, Cornish KG, Patel KP (2003) Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res 93(10):990–997. doi:10.1161/01.RES.0000102865.60437.55
Oldfield BJ, Davern PJ, Giles ME, Allen AM, Badoer E, McKinley MJ (2001) Efferent neural projections of angiotensin receptor (AT1) expressing neurones in the hypothalamic paraventricular nucleus of the rat. J Neuroendocrinol 13(2):139–146
Herman JP, Eyigor O, Ziegler DR, Jennes L (2000) Expression of ionotropic glutamate receptor subunit mRNAs in the hypothalamic paraventricular nucleus of the rat. J Comp Neurol 422(3):352–362
Latchford KJ, Ferguson AV (2005) Angiotensin depolarizes parvocellular neurons in paraventricular nucleus through modulation of putative nonselective cationic and potassium conductances. Am J Physiol Regul Integr Comp Physiol 289(1):R52–R58. doi:10.1152/ajpregu.00549.2004
Wei SG, Yu Y, Zhang ZH, Weiss RM, Felder RB (2008) Mitogen-activated protein kinases mediate upregulation of hypothalamic angiotensin II type 1 receptors in heart failure rats. Hypertension 52(4):679–686. doi:10.1161/HYPERTENSIONAHA.108.113639
Sharma NM, Zheng H, Mehta PP, Li YF, Patel KP (2011) Decreased nNOS in the PVN leads to increased sympathoexcitation in chronic heart failure: role for CAPON and Ang II. Cardiovasc Res 92(2):348–357. doi:10.1093/cvr/cvr217
Li DP, Yang Q, Pan HM, Pan HL (2008) Plasticity of pre- and postsynaptic GABAB receptor function in the paraventricular nucleus in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 295(2):H807–H815. doi:10.1152/ajpheart.00259.2008
Park JB, Skalska S, Son S, Stern JE (2007) Dual GABAA receptor-mediated inhibition in rat presympathetic paraventricular nucleus neurons. J Physiol 582(Pt 2):539–551. doi:10.1113/jphysiol.2007.133223
Li DP, Pan HL (2007) Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther 320(2):615–626. doi:10.1124/jpet.106.109538
Cork SC, Chazot PL, Pyner S (2010) NMDA receptor subunit expression in the paraventricular nucleus of the spontaneously hypertensive (SHR) and pregnant rat. Proc Physiol Soc 21, PC32
Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C (1997) Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol 18(4):383–439. doi:10.1006/frne1997.0155
Li Z, Ferguson AV (1993) Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol 265(2 Pt 2):R302–R309
Llewellyn T, Zheng H, Liu X, Xu B, Patel KP (2012) Median preoptic nucleus and subfornical organ drive renal sympathetic nerve activity via a glutamatergic mechanism within the paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 302(4):R424–R432. doi:10.1152/ajpregu.00403.2011
Benarroch EE (2005) Paraventricular nucleus, stress response, and cardiovascular disease. Clin Auton Res 15(4):254–263. doi:10.1007/s10286-005-0290-7
Latchford KJ, Ferguson AV (2004) ANG II-induced excitation of paraventricular nucleus magnocellular neurons: a role for glutamate interneurons. Am J Physiol Regul Integr Comp Physiol 286(5):R894–R902. doi:10.1152/ajpregu.00603.2003
Coleman CG, Anrather J, Iadecola C, Pickel VM (2009) Angiotensin II type 2 receptors have a major somatodendritic distribution in vasopressin-containing neurons in the mouse hypothalamic paraventricular nucleus. Neuroscience 163(1):129–142. doi:10.1016/j.neuroscience.2009.06.032
Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH (2004) Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95(9):937–944. doi:10.1161/01.RES.0000146676.04359.64
Han Y, Yuan N, Zhang SJ, Gao J, Shi Z, Zhou YB, Gao XY, Zhu GQ (2011) c-Src in paraventricular nucleus modulates sympathetic activity and cardiac sympathetic afferent reflex in renovascular hypertensive rats. Pflugers Arch 461(4):437–446. doi:10.1007/s00424-011-0932-7
Chen Q, Pan HL (2007) Signaling mechanisms of angiotensin II-induced attenuation of GABAergic input to hypothalamic presympathetic neurons. J Neurophysiol 97(5):3279–3287. doi:10.1152/jn.01329.2006
Yu Y, Zhong MK, Li J, Sun XL, Xie GQ, Wang W, Zhu GQ (2007) Endogenous hydrogen peroxide in paraventricular nucleus mediating cardiac sympathetic afferent reflex and regulating sympathetic activity. Pflugers Arch 454(4):551–557. doi:10.1007/s00424-007-0256-9
Johns DW, Peach MJ, Gomez RA, Inagami T, Carey RM (1990) Angiotensin II regulates renin gene expression. Am J Physiol 259(6 Pt 2):F882–F887
Wei SG, Yu Y, Zhang ZH, Felder RB (2009) Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J Physiol Heart Circ Physiol 296(5):H1425–H1433. doi:10.1152/ajpheart.00942.2008
Leclerc PC, Lanctot PM, Auger-Messier M, Escher E, Leduc R, Guillemette G (2006) S-nitrosylation of cysteine 289 of the AT1 receptor decreases its binding affinity for angiotensin II. Br J Pharmacol 148(3):306–313. doi:10.1038/sj.bjp.0706725
Sharma NM, Llewellyn TL, Zheng H, Patel KP (2013) Angiotensin II-mediated posttranslational modification of nNOS in the PVN of rats with CHF: role for PIN. Am J Physiol Heart Circ Physiol 305(6):H843–H855. doi:10.1152/ajpheart.00170.2013
Wegener G, Harvey BH, Bonefeld B, Muller HK, Volke V, Overstreet DH, Elfving B (2010) Increased stress-evoked nitric oxide signalling in the Flinders sensitive line (FSL) rat: a genetic animal model of depression. Int J Neuropsychopharmacol 13(4):461–473. doi:10.1017/s1461145709990241
Xu B, Wratten N, Charych EI, Buyske S, Firestein BL, Brzustowicz LM (2005) Increased expression in dorsolateral prefrontal cortex of CAPON in schizophrenia and bipolar disorder. PLoS Med 2(10):e263. doi:10.1371/journal.pmed.0020263
Gao L, Zucker IH (2011) AT2 receptor signaling and sympathetic regulation. Curr Opin Pharmacol 11(2):124–130. doi:10.1016/j.coph.2010.11.004
Zheng H, Liu X, Patel KP (2011) Angiotensin-converting enzyme 2 overexpression improves central nitric oxide-mediated sympathetic outflow in chronic heart failure. Am J Physiol Heart Circ Physiol 301(6):H2402–H2412. doi:10.1152/ajpheart.00330.2011
Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB (2008) Does aldosterone upregulate the brain renin–angiotensin system in rats with heart failure? Hypertension 51(3):727–733. doi:10.1161/HYPERTENSIONAHA.107.099796
Ye P, Kenyon CJ, Mackenzie SM, Nichol K, Seckl JR, Fraser R, Connell JM, Davies E (2008) Effects of ACTH, dexamethasone, and adrenalectomy on 11beta-hydroxylase (CYP11B1) and aldosterone synthase (CYP11B2) gene expression in the rat central nervous system. J Endocrinol 196(2):305–311. doi:10.1677/JOE-07-0439
Huang BS, Zheng H, Tan J, Patel KP, Leenen FH (2011) Regulation of hypothalamic renin–angiotensin system and oxidative stress by aldosterone. Exp Physiol 96(10):1028–1038. doi:10.1113/expphysiol.2011.059840
Zhang ZH, Yu Y, Wei SG, Felder RB (2012) Aldosterone-induced brain MAPK signaling and sympathetic excitation are angiotensin II type-1 receptor dependent. Am J Physiol Heart Circ Physiol 302(3):H742–H751. doi:10.1152/ajpheart.00856.2011
Xue B, Beltz TG, Yu Y, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK (2011) Central interactions of aldosterone and angiotensin II in aldosterone- and angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 300(2):H555–H564. doi:10.1152/ajpheart.00847.2010
Chen J, Gomez-Sanchez CE, Penman A, May PJ, Gomez-Sanchez EP (2013) Expression of mineralocorticoid and glucocorticoid receptors in pre-autonomic neurons of the rat paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. doi:10.1152/ajpregu.00506.2013
Geerling JC, Loewy AD (2009) Aldosterone in the brain. Am J Physiol Renal Physiol 297(3):F559–F576. doi:10.1152/ajprenal.90399.2008
Gomez-Sanchez EP, Venkataraman MT, Thwaites D, Fort C (1990) ICV infusion of corticosterone antagonizes ICV-aldosterone hypertension. Am J Physiol 258(4 Pt 1):E649–E653
Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB (2008) Aldosterone acts centrally to increase brain renin–angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol 294(2):H1067–H1074. doi:10.1152/ajpheart.01131.2007
Damas JK, Gullestad L, Aukrust P (2001) Cytokines as new treatment targets in chronic heart failure. Curr Control Trials Cardiovasc Med 2(6):271–277
Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J (2008) Cross-talk between cytokines and renin–angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-kappaB. Cardiovasc Res 79(4):671–678. doi:10.1093/cvr/cvn119
Yu Y, Zhang ZH, Wei SG, Chu Y, Weiss RM, Heistad DD, Felder RB (2007) Central gene transfer of interleukin-10 reduces hypothalamic inflammation and evidence of heart failure in rats after myocardial infarction. Circ Res 101(3):304–312. doi:10.1161/CIRCRESAHA.107.148940
Shi P, Raizada MK, Sumners C (2010) Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharmacol Physiol 37(2):e52–e57. doi:10.1111/j.1440-1681.2009.05234.x
Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB (2006) Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res 99(7):758–766. doi:10.1161/01.RES.0000244092.95152.86
Buller KM (2001) Role of circumventricular organs in pro-inflammatory cytokine-induced activation of the hypothalamic-pituitary-adrenal axis. Clin Exp Pharmacol Physiol 28(7):581–589
Ericsson A, Arias C, Sawchenko PE (1997) Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. The J Neurosci 17(18):7166–7179
Kapcala LP, He JR, Gao Y, Pieper JO, DeTolla LJ (1996) Subdiaphragmatic vagotomy inhibits intra-abdominal interleukin-1 beta stimulation of adrenocorticotropin secretion. Brain Res 728(2):247–254
Katsuura G, Gottschall PE, Dahl RR, Arimura A (1988) Adrenocorticotropin release induced by intracerebroventricular injection of recombinant human interleukin-1 in rats: possible involvement of prostaglandin. Endocrinology 122(5):1773–1779. doi:10.1210/endo-122-5-1773
Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB (2013) Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension 62(1):118–125. doi:10.1161/HYPERTENSIONAHA.113.01404
Zhang ZH, Yu Y, Wei SG, Nakamura Y, Nakamura K, Felder RB (2011) EP(3) receptors mediate PGE(2)-induced hypothalamic paraventricular nucleus excitation and sympathetic activation. Am J Physiol Heart Circ Physiol 301(4):H1559–H1569. doi:10.1152/ajpheart.00262.2011
Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB (2010) Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension 55(3):652–659. doi:10.1161/HYPERTENSIONAHA.109.142836
Mulla A, Buckingham JC (1999) Regulation of the hypothalamo-pituitary-adrenal axis by cytokines. Baillieres Best Pract Res Clin Endocrinol Metab 13(4):503–521
Yu XJ, Suo YP, Qi J, Yang Q, Li HH, Zhang DM, Yi QY, Zhang J, Zhu GQ, Zhu Z, Kang YM (2013) Interaction between AT1 receptor and NF-kappaB in hypothalamic paraventricular nucleus contributes to oxidative stress and sympathoexcitation by modulating neurotransmitters in heart failure. Cardiovasc Toxicol 13(4):381–390. doi:10.1007/s12012-013-9219-x
Dekker RL, Moser DK, Tovar EG, Chung ML, Heo S, Wu JR, Dunbar SB, Pressler SJ, Lennie TA (2013) Depressive symptoms and inflammatory biomarkers in patients with heart failure. Eur J Cardiovasc Nurs. doi:10.1177/1474515113507508
Grippo AJ, Francis J, Weiss RM, Felder RB, Johnson AK (2003) Cytokine mediation of experimental heart failure-induced anhedonia. Am J Physiol Regul Integr Comp Physiol 284(3):R666–R673
Ferketich AK, Ferguson JP, Binkley PF (2005) Depressive symptoms and inflammation among heart failure patients. Am Heart J 150(1):132–136. doi:10.1016/j.ahj.2004.08.029
Moorman AJ, Mozaffarian D, Wilkinson CW, Lawler RL, McDonald GB, Crane BA, Spertus JA, Russo JE, Stempien-Otero AS, Sullivan MD, Levy WC (2007) In patients with heart failure elevated soluble TNF-receptor 1 is associated with higher risk of depression. J Card Fail 13(9):738–743. doi:10.1016/j.cardfail.2007.06.301
Kupper N, Widdershoven JW, Pedersen SS (2012) Cognitive/affective and somatic/affective symptom dimensions of depression are associated with current and future inflammation in heart failure patients. J Affect Disord 136(3):567–576. doi:10.1016/j.jad.2011.10.029
Moughrabi S, Evangelista LS, Habib SI, Kassabian L, Breen EC, Nyamathi A, Irwin M (2013) In patients with stable heart failure, soluble TNF-receptor 2 is associated with increased risk for depressive symptoms. Biol Res Nurs. doi:10.1177/1099800413496454
Benicky J, Sanchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang DM, Saavedra JM (2011) Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology 36(4):857–870. doi:10.1038/npp.2010.225
Saavedra JM (2012) Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin Sci (Lond) 123(10):567–590. doi:10.1042/cs20120078
Almeida OP, Garrido GJ, Etherton-Beer C, Lautenschlager NT, Arnolda L, Alfonso H, Flicker L (2013) Brain and mood changes over 2 years in healthy controls and adults with heart failure and ischaemic heart disease. Eur J Heart Fail 15(8):850–858. doi:10.1093/eurjhf/hft029
Almeida OP, Garrido GJ, Beer C, Lautenschlager NT, Arnolda L, Flicker L (2012) Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur Heart J 33(14):1769–1776. doi:10.1093/eurheartj/ehr467
Pan A, Kumar R, Macey PM, Fonarow GC, Harper RM, Woo MA (2013) Visual assessment of brain magnetic resonance imaging detects injury to cognitive regulatory sites in patients with heart failure. J Card Fail 19(2):94–100. doi:10.1016/j.cardfail.2012.12.001
Bernstein HG, Klix M, Dobrowolny H, Brisch R, Steiner J, Bielau H, Gos T, Bogerts B (2012) A postmortem assessment of mammillary body volume, neuronal number and densities, and fornix volume in subjects with mood disorders. Eur Arch Psychiatry Clin Neurosci 262(8):637–646. doi:10.1007/s00406-012-0300-4
Pyner S (2009) Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat 38(3):197–208. doi:10.1016/j.jchemneu.2009.03.005
Kalra A, Maharaj V, Goldsmith SR (2013) Vasopressin receptor antagonists: from pivotal trials to current practice. Curr Heart Fail Rep. doi:10.1007/s11897-013-0175-3
Sivukhina EV, Morozov IuE, Dolzhikov AA, Jirikowski GF, Grinevich V (2010) Comparison of vasopressin and oxytocin expressions in the hypothalamo-neurohypophysial system of patients with chronic heart failure. Horm Metab Res 42(1):56–60. doi:10.1055/s-0029-1234081
Antunes VR, Yao ST, Pickering AE, Murphy D, Paton JF (2006) A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation. J Physiol 576(Pt 2):569–583. doi:10.1113/jphysiol.2006.115766
Motawei K, Pyner S, Ranson RN, Kamel M, Coote JH (1999) Terminals of paraventricular spinal neurones are closely associated with adrenal medullary sympathetic preganglionic neurones: immunocytochemical evidence for vasopressin as a possible neurotransmitter in this pathway. Exp Brain Res 126(1):68–76
Swanson LW, McKellar S (1979) The distribution of oxytocin- and neurophysin-stained fibers in the spinal cord of the rat and monkey. J Comp Neurol 188(1):87–106. doi:10.1002/cne.901880108
Daftary SS, Boudaba C, Tasker JG (2000) Noradrenergic regulation of parvocellular neurons in the rat hypothalamic paraventricular nucleus. Neuroscience 96(4):743–751
Basu S, Sinha SK, Shao Q, Ganguly PK, Dhalla NS (1996) Neuropeptide Y modulation of sympathetic activity in myocardial infarction. J Am Coll Cardiol 27(7):1796–1803
Kawabe T, Kawabe K, Sapru HN (2012) Cardiovascular responses to chemical stimulation of the hypothalamic arcuate nucleus in the rat: role of the hypothalamic paraventricular nucleus. PLoS ONE 7(9):e45180. doi:10.1371/journal.pone.0045180
Parissis JT, Farmakis D, Fountoulaki K, Rigas A, Nikolaou M, Paraskevaidis IA, Bistola V, Venetsanou K, Ikonomidis I, Anastasiou-Nana M, Kremastinos DT, Filippatos G (2013) Clinical and neurohormonal correlates and prognostic value of serum prolactin levels in patients with chronic heart failure. Eur J Heart Fail 15(10):1122–1130. doi:10.1093/eurjhf/hft070
Ito K, Hirooka Y, Matsukawa R, Nakano M, Sunagawa K (2012) Decreased brain sigma-1 receptor contributes to the relationship between heart failure and depression. Cardiovasc Res 93(1):33–40. doi:10.1093/cvr/cvr255
Saavedra JM, Chevillard C (1982) Angiotensin-converting enzyme is present in the subfornical organ and other circumventricular organs of the rat. Neurosci Lett 29(2):123–127
Sakaguchi K, Chai SY, Jackson B, Johnston CI, Mendelsohn FA (1988) Inhibition of tissue angiotensin converting enzyme. Quantitation by autoradiography. Hypertension 11(3):230–238
Chai SY, Mendelsohn FA, Paxinos G (1987) Angiotensin converting enzyme in rat brain visualized by quantitative in vitro autoradiography. Neuroscience 20(2):615–627
Sakaguchi K, Chai SY, Jackson B, Johnston CI, Mendelsohn FA (1987) Blockade of angiotensin converting enzyme in circumventricular organs of the brain after oral lisinopril administration demonstrated by quantitative in vitro autoradiography. Clin Exp Pharmacol Physiol 14(3):155–158
McKinley MJ, Colvill LM, Giles ME, Oldfield BJ (1997) Distribution of Fos-immunoreactivity in rat brain following a dipsogenic dose of captopril and effects of angiotensin receptor blockade. Brain Res 747(1):43–51
Wang JM, Tan J, Leenen FH (2003) Central nervous system blockade by peripheral administration of AT1 receptor blockers. J Cardiovasc Pharmacol 41(4):593–599
Pavel J, Benicky J, Murakami Y, Sanchez-Lemus E, Saavedra JM (2008) Peripherally administered angiotensin II AT1 receptor antagonists are anti-stress compounds in vivo. Ann N Y Acad Sci 1148:360–366. doi:10.1196/annals.1410.006
Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, Riegger GA, Malbecq W, Smith RD, Guptha S, Poole-Wilson PA (2009) Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet 374(9704):1840–1848. doi:10.1016/S0140-6736(09)61913-9
Ruzicka M, Floras JS, McReynolds AJ, Coletta E, Haddad H, Davies R, Leenen FH (2013) Do high doses of AT(1)-receptor blockers attenuate central sympathetic outflow in humans with chronic heart failure? Clin Sci (Lond) 124(9):589–595. doi:10.1042/CS20120437
McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA, Investigators C, Committees (2003) Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 362(9386):767–771. doi:10.1016/S0140-6736(03)14283-3
Ma TK, Kam KK, Yan BP, Lam YY (2010) Renin–angiotensin–aldosterone system blockade for cardiovascular diseases: current status. Br J Pharmacol 160(6):1273–1292. doi:10.1111/j.1476-5381.2010.00750.x
Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B (2011) Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 364(1):11–21. doi:10.1056/NEJMoa1009492
Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M (2003) Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348(14):1309–1321. doi:10.1056/NEJMoa030207
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341(10):709–717. doi:10.1056/NEJM199909023411001
Lal A, Veinot JP, Leenen FH (2004) Critical role of CNS effects of aldosterone in cardiac remodeling post-myocardial infarction in rats. Cardiovasc Res 64(3):437–447. doi:10.1016/j.cardiores.2004.08.004
Francis J, Weiss RM, Johnson AK, Felder RB (2003) Central mineralocorticoid receptor blockade decreases plasma TNF-alpha after coronary artery ligation in rats. Am J Physiol Regul Integr Comp Physiol 284(2):R328–R335. doi:10.1152/ajpregu.00376.2002
Lal A, Veinot JP, Ganten D, Leenen FH (2005) Prevention of cardiac remodeling after myocardial infarction in transgenic rats deficient in brain angiotensinogen. J Mol Cell Cardiol 39(3):521–529. doi:10.1016/j.yjmcc.2005.05.002
Shaw SM, Shah MK, Williams SG, Fildes JE (2009) Immunological mechanisms of pentoxifylline in chronic heart failure. Eur J Heart Fail 11(2):113–118. doi:10.1093/eurjhf/hfn040
Vakili A, Zahedi khorasani M (2007) Post-ischemic treatment of pentoxifylline reduces cortical not striatal infarct volume in transient model of focal cerebral ischemia in rat. Brain Res 1144:186–191. doi:10.1016/j.brainres.2007.01.096
Sliwa K, Skudicky D, Candy G, Wisenbaugh T, Sareli P (1998) Randomised investigation of effects of pentoxifylline on left-ventricular performance in idiopathic dilated cardiomyopathy. Lancet 351(9109):1091–1093. doi:10.1016/S0140-6736(97)09338-0
Skudicky D, Sliwa K, Bergemann A, Candy G, Sareli P (2000) Reduction in Fas/APO-1 plasma concentrations correlates with improvement in left ventricular function in patients with idiopathic dilated cardiomyopathy treated with pentoxifylline. Heart 84(4):438–439
Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T (2004) Targeted anticytokine therapy in patients with chronic heart failure: results of the randomized etanercept worldwide evaluation (RENEWAL). Circulation 109(13):1594–1602. doi:10.1161/01.CIR.0000124490.27666.B2
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT (2003) Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107(25):3133–3140. doi:10.1161/01.CIR.0000077913.60364.D2
Javed Q, Murtaza I (2013) Therapeutic potential of tumour necrosis factor-alpha antagonists in patients with chronic heart failure. Heart Lung Circ 22(5):323–327. doi:10.1016/j.hlc.2012.12.002
Van Tassell BW, Arena R, Biondi-Zoccai G, McNair Canada J, Oddi C, Abouzaki NA, Jahangiri A, Falcao RA, Kontos MC, Shah KB, Voelkel NF, Dinarello CA, Abbate A (2013) Effects of interleukin-1 blockade with Anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART Pilot Study). Am J Cardiol. doi:10.1016/j.amjcard.2013.08.047
Dinarello CA, van der Meer JW (2013) Treating inflammation by blocking interleukin-1 in humans. Semin Immunol. doi:10.1016/j.smim.2013.10.008
Galea J, Ogungbenro K, Hulme S, Greenhalgh A, Aarons L, Scarth S, Hutchinson P, Grainger S, King A, Hopkins SJ, Rothwell N, Tyrrell P (2011) Intravenous anakinra can achieve experimentally effective concentrations in the central nervous system within a therapeutic time window: results of a dose-ranging study. J Cerebral Blood Flow Metab 31(2):439–447. doi:10.1038/jcbfm.2010.103
Pathan SA, Iqbal Z, Zaidi SM, Talegaonkar S, Vohra D, Jain GK, Azeem A, Jain N, Lalani JR, Khar RK, Ahmad FJ (2009) CNS drug delivery systems: novel approaches. Recent Pat Drug Deliv Formul 3(1):71–89
Philippens IH, Wubben JA, Finsen B, t Hart BA (2013) Oral treatment with the NADPH oxidase antagonist apocynin mitigates clinical and pathological features of parkinsonism in the MPTP marmoset model. J Neuroimmune Pharmacol 8(3):715–726. doi:10.1007/s11481-013-9450-z
Gomes ME, El Messaoudi S, Lenders JW, Bellersen L, Verheugt FW, Smits P, Tack CJ (2011) High dose ascorbic acid does not reverse central sympathetic overactivity in chronic heart failure. J Clin Pharm Ther 36(5):546–552. doi:10.1111/j.1365-2710.2010.01205.x
Lindley TE, Infanger DW, Rishniw M, Zhou Y, Doobay MF, Sharma RV, Davisson RL (2009) Scavenging superoxide selectively in mouse forebrain is associated with improved cardiac function and survival following myocardial infarction. Am J Physiol Regul Integr Comp Physiol 296(1):R1–R8. doi:10.1152/ajpregu.00078.2008
Yu Y, Zhang ZH, Wei SG, Weiss RM, Felder RB (2012) Peroxisome proliferator-activated receptor-gamma regulates inflammation and renin–angiotensin system activity in the hypothalamic paraventricular nucleus and ameliorates peripheral manifestations of heart failure. Hypertension 59(2):477–484. doi:10.1161/HYPERTENSIONAHA.111.182345
Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, Griffin PR, Spiegelman BM (2010) Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature 466(7305):451–456. doi:10.1038/nature09291
Yakisich JS, Vita MF, Siden A, Tasat DR, Cruz M (2010) Strong inhibition of replicative DNA synthesis in the developing rat cerebral cortex and glioma cells by roscovitine. Invest New Drugs 28(3):299–305. doi:10.1007/s10637-009-9254-4
Kabadi SV, Stoica BA, Byrnes KR, Hanscom M, Loane DJ, Faden AI (2012) Selective CDK inhibitor limits neuroinflammation and progressive neurodegeneration after brain trauma. J Cereb Blood Flow Metab 32(1):137–149. doi:10.1038/jcbfm.2011.117
Wang J, Pang T, Hafko R, Benicky J, Sanchez-Lemus E, Saavedra JM (2013) Telmisartan ameliorates glutamate-induced neurotoxicity: roles of AT receptor blockade and PPARgamma activation. Neuropharmacology 79C:249–261. doi:10.1016/j.neuropharm.2013.11.022
Kompa AR, See F, Lewis DA, Adrahtas A, Cantwell DM, Wang BH, Krum H (2008) Long-term but not short-term p38 mitogen-activated protein kinase inhibition improves cardiac function and reduces cardiac remodeling post-myocardial infarction. J Pharmacol Exp Ther 325(3):741–750. doi:10.1124/jpet.107.133546
Fijen JW, Zijlstra JG, De Boer P, Spanjersberg R, Tervaert JW, Van Der Werf TS, Ligtenberg JJ, Tulleken JE (2001) Suppression of the clinical and cytokine response to endotoxin by RWJ-67657, a p38 mitogen-activated protein-kinase inhibitor, in healthy human volunteers. Clin Exp Immunol 124(1):16–20
Kimmel SE, Schelleman H, Berlin JA, Oslin DW, Weinstein RB, Kinman JL, Sauer WH, Lewis JD (2011) The effect of selective serotonin re-uptake inhibitors on the risk of myocardial infarction in a cohort of patients with depression. Br J Clin Pharmacol 72(3):514–517. doi:10.1111/j.1365-2125.2011.04008.x
Davies SJ, Hood SD, Argyropoulos SV, Morris K, Bell C, Witchel HJ, Jackson PR, Nutt DJ, Potokar JP (2006) Depleting serotonin enhances both cardiovascular and psychological stress reactivity in recovered patients with anxiety disorders. J Clin Psychopharmacol 26(4):414–418. doi:10.1097/01.jcp.0000227704.79740.c0
Leftheriotis D, Flevari P, Ikonomidis I, Douzenis A, Liapis C, Paraskevaidis I, Iliodromitis E, Lykouras L, Kremastinos DT (2010) The role of the selective serotonin re-uptake inhibitor sertraline in nondepressive patients with chronic ischemic heart failure: a preliminary study. Pacing Clin Electrophysiol 10:1217–1223. doi:10.1111/j.1540-8159.2010.02792.x
Henze M, Tiniakov R, Samarel A, Holmes E, Scrogin K (2013) Chronic fluoxetine reduces autonomic control of cardiac rhythms in rats with congestive heart failure. Am J Physiol Heart Circ Physiol 304(3):H444–H454. doi:10.1152/ajpheart.00763.2012
Schmieder RE, Redon J (2012) Comment on ESH position paper: renal denervation—an interventional therapy of resistant hypertension. J Hypertens 30(12):2443. doi:10.1097/HJH.0b013e3283599beb
Doumas M, Faselis C, Papademetriou V (2010) Renal sympathetic denervation and systemic hypertension. Am J Cardiol 105(4):570–576. doi:10.1016/j.amjcard.2009.10.027
Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, Hamady M, Hughes AD, Sever PS, Sobotka PA, Francis DP (2013) First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol 162(3):189–192. doi:10.1016/j.ijcard.2012.09.019
Lim GB (2012) Hypertension. Cardiac effects of renal denervation. Nat Rev Cardiol 9(5):256. doi:10.1038/nrcardio.2012.39
Schiller AM, Haack KK, Pellegrino PR, Curry PL, Zucker IH (2013) Unilateral renal denervation improves autonomic balance in conscious rabbits with chronic heart failure. Am J Physiol Regul Integr Comp Physiol 305(8):R886–R892. doi:10.1152/ajpregu.00269.2013
O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL (2009) Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301(14):1439–1450. doi:10.1001/jama.2009.454
O’Connor CM, Mentz RJ, Whellan DJ (2011) Covariate adjustment in heart failure randomized controlled clinical trials: a case analysis of the HF-ACTION trial. Heart Fail Clin 7(4):497–500. doi:10.1016/j.hfc.2011.06.011
Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrao CE (2003) The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol 42(5):854–860
Patel KP, Zheng H (2012) Central neural control of sympathetic nerve activity in heart failure following exercise training. Am J Physiol Heart Circ Physiol 302(3):H527–H537. doi:10.1152/ajpheart.00676.2011
Acknowledgments
This work was supported by the Portuguese Foundation for Science and Technology [Grants PEst-C/SAU/UI0051/2011 and EXCL/BIM-MEC/0055/2012, partially funded by FEDER through COMPETE] through the Cardiovascular R&D Unit and by European Commission [Grant FP7-Health-2010; MEDIA-261409].
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sousa-Pinto, B., Ferreira-Pinto, M.J., Santos, M. et al. Central nervous system circuits modified in heart failure: pathophysiology and therapeutic implications. Heart Fail Rev 19, 759–779 (2014). https://doi.org/10.1007/s10741-014-9427-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-014-9427-x