Abstract
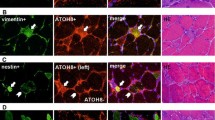
Recent study has reported that α7 nicotine acetylcholine receptor (α7nAChR) is expressed in regenerated multinucleated myotubes. But the distribution of α7nAChR in satellite cells in different myogenic status is unknown. A preliminary study on the dynamic distribution of α7nAChR in satellite cells was performed by double indirect immunofluorescent procedures during skeletal muscle wound healing in rats. An animal model of skeletal muscle contusion was established in 40 Sprague–Dawley male rats. Samples were taken at 1, 3, 5, 7, 9, 13, 17 and 21 days after injury, respectively (five rats in each posttraumatic interval). Five rats were employed as control. In normal muscle specimens, weak immunoreactivity for α7nAChR was detected in a few satellite cells (considered as quiescent). α7nAChR-positive signals were observed in proliferated and differentiated satellite cells and regenerated multinucleated myotubes in the wounded areas. By morphometric analysis, the average number of α7nAChR+/Pax7+ and α7nAChR+/MyoD+ cells climaxed at 5 days post-injury. The average number of α7nAChR+/myogenin+ cells was significantly increased from 3 to 9 days post-injury as compared with other posttraumatic intervals. The protein level of α7nAChR maximized at 9 days post-injury, which implies that α7nAChR was associated with the satellite cells status. Our observations on expression of α7nAChR in satellite cells from quiescence to myotube formation suggest that α7nAChR may be involved in muscle regeneration by regulating satellite cell status.








Similar content being viewed by others
References
Baoge L, Van Den Steen E, Rimbaut S, Philips N, Witvrouw E, Almqvist KF, Vanderstraeten G, Vanden Bossche LC (2012) Treatment of skeletal muscle injury: a review. ISRN Orthop 2012:689012
Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238
Colon-Saez JO, Yakel JL (2014) A mutation in the extracellular domain of the alpha7 nAChR reduces calcium permeability. Pflugers Arch 466:1571–1579
Costa R, Motta EM, Manjavachi MN, Cola M, Calixto JB (2012) Activation of the alpha-7 nicotinic acetylcholine receptor (alpha7 nAchR) reverses referred mechanical hyperalgesia induced by colonic inflammation in mice. Neuropharmacology 63:798–805
Creuzet S, Lescaudron L, Li Z, Fontaine-Perus J (1998) MyoD, myogenin, and desmin-nls-lacZ transgene emphasize the distinct patterns of satellite cell activation in growth and regeneration. Exp Cell Res 243:241–253
de Jonge WJ, Ulloa L (2007) The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 151:915–929
Diao Y, Guo X, Li Y, Sun K, Lu L, Jiang L, Fu X, Zhu H, Sun H, Wang H, Wu Z (2012) Pax3/7BP is a Pax7-and Pax3-binding protein that regulates the proliferation of muscle precursor cells by an epigenetic mechanism. Cell Stem Cell 11:231–241
Fan YY, Zhang ST, Yu LS, Ye GH, Lin KZ, Wu SZ, Dong MW, Han JG, Feng XP, Li XB (2014) The time-dependent expression of alpha7nAChR during skeletal muscle wound healing in rats. Int J Legal Med 128:779–786
Figeac N, Serralbo O, Marcelle C, Zammit PS (2014) ErbB3 binding protein-1 (Ebp1) controls proliferation and myogenic differentiation of muscle stem cells. Dev Biol 386:135–151
Grounds MD, Garrett KL, Lai MC, Wright WE, Beilharz MW (1992) Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res 267:99–104
Hatade T, Takeuchi K, Fujita N, Arakawa T, Miki A (2014) Effect of heat stress soon after muscle injury on the expression of MyoD and myogenin during regeneration process. J Musculoskelet Neuronal Interact 14:325–333
Holterman CE, Rudnicki MA (2005) Molecular regulation of satellite cell function. Semin Cell Dev Biol 16:575–584
Järvinen TA, Järvinen TL, Kääriäinen M, Kalimo H, Järvinen M (2005) Muscle injuries biology and treatment. Am J Sports Med 33:745–764
Karalaki M, Fili S, Philippou A, Koutsilieris M (2009) Muscle regeneration: cellular and molecular events. In Vivo 23:779–796
Kelso ML, Oestreich JH (2012) Traumatic brain injury: central and peripheral role of alpha7 nicotinic acetylcholine receptors. Curr Drug Targets 13:631–636
Koishi K, Zhang M, McLennan IS, Harris AJ (1995) MyoD protein accumulates in satellite cells and is neurally regulated in regenerating myotubes and skeletal muscle fibers. Dev Dyn 202:244–254
Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA (2007) The non-neuronal cholinergic system of human skin. Horm Metab Res 39:125–135
Lawson-Smith MJ, McGeachie JK (1998) The identification of myogenic cells in skeletal muscle, with emphasis on the use of tritiated thymidine autoradiography and desmin antibodies. J Anat 192:161–171
Legerlotz K, Smith HK (2008) Role of MyoD in denervated, disused, and exercised muscle. Muscle Nerve 38:1087–1100
Lin W, Hirata N, Sekino Y, Kanda Y (2012) Role of alpha7-nicotinic acetylcholine receptor in normal and cancer stem cells. Curr Drug Targets 13:656–665
Marg A, Escobar H, Gloy S, Kufeld M, Zacher J, Spuler A, Birchmeier C, Izsvák Z, Spuler S (2014) Human satellite cells have regenerative capacity and are genetically manipulable. J Clin Invest 124:4257–4265
Marrero MB, Bencherif M, Lippiello PM, Lucas R (2011) Application of alpha7 nicotinic acetylcholine receptor agonists in inflammatory diseases: an overview. Pharm Res 28:413–416
Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA (1996) MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev 10:1173–1183
Mokalled MH, Johnson AN, Creemers EE, Olson EN (2012) MASTR directs MyoD-dependent satellite cell differentiation during skeletal muscle regeneration. Genes Dev 26:190–202
Motohashi N, Asakura A (2014) Muscle satellite cell heterogeneity and self-renewal. Front Cell Dev Biol 2:1
Ogura Y, Mishra V, Hindi SM, Kuang S, Kumar A (2013) Proinflammatory cytokine tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) suppresses satellite cell self-renewal through inversely modulating notch and NF-κB signaling pathways. J Biol Chem 288:35159–35169
Olguin HC, Olwin BB (2004) Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol 275:375–388
Powell DJ, McFarland DC, Cowieson AJ, Muir WI, Velleman SG (2014) The effect of nutritional status on myogenic gene expression of satellite cells derived from different muscle types. Poult Sci 93:2278–2288
Rahman MM, Ghosh M, Subramani J, Fong GH, Carlson ME, Shapiro LH (2014) CD13 regulates anchorage and differentiation of the skeletal muscle satellite stem cell population in ischemic injury. Stem Cells 32:1564–1577
Razani-Boroujerdi S, Boyd RT, Dávila-García MI, Nandi JS, Mishra NC, Singh SP, Pena-Philippides JC, Langley R, Sopori ML (2007) T cells express alpha7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J Immunol 179:2889–2898
Stegemann A, Sindrilaru A, Eckes B, del Rey A, Heinick A, Schulte JS, Müller FU, Grando SA, Fiebich BL, Scharffetter-Kochanek K, Luger TA, Böhm M (2013) Tropisetron suppresses collagen synthesis in skin fibroblasts via alpha7 nicotinic acetylcholine receptor and attenuates fibrosis in a scleroderma mouse model. Arthritis Rheum 65:792–804
Taly A, Charon S (2012) Alpha7 nicotinic acetylcholine receptors: a therapeutic target in the structure era. Curr Drug Targets 13:695–706
Thomsen MS, Mikkelsen JD (2012) The alpha7 nicotinic acetylcholine receptor complex: one, two or multiple drug targets? Curr Drug Targets 13:707–720
von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA (2013) Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci USA 110:16474–16479
Wang YX, Rudnicki MA (2011) Satellite cells, the engines of muscle repair. Nat Rev Mol Cell Biol 13:127–133
Yu TS, Cheng ZH, Li LQ, Zhao R, Fan YY, Du Y, Ma WX, Guan DW (2010) The cannabinoid receptor type 2 is time-dependently expressed during skeletal muscle wound healing in rats. Int J Legal Med 124:397–404
Zammit PS, Partridge TA, Yablonka-Reuveni Z (2006) The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem 54:1177–1191
Zhang ST, Zhao R, Ma WX, Fan YY, Guan WZ, Wang J, Ren P, Zhong K, Yu TS, Pi JB, Guan DW (2013) Nrf1 is time-dependently expressed and distributed in the distinct cell types after trauma to skeletal muscles in rats. Histol Histopathol 28:725–735
Acknowledgments
The study was financially supported in part by grants from research fund for the Doctoral Program funded by Ministry of Education of China (20122104110025) and from projects funded by National Natural Science Foundation of China (81273342) and Shenyang Science and Technology Bureau (F12-277-1-03).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tian, ZL., Jiang, SK., Zhang, M. et al. α7nAChR is expressed in satellite cells at different myogenic status during skeletal muscle wound healing in rats. J Mol Hist 46, 499–509 (2015). https://doi.org/10.1007/s10735-015-9641-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-015-9641-4