Abstract
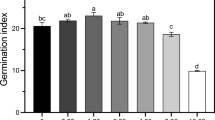
Salicylic acid (SA) can alleviate damage to rice plants induced by heat stress, but its role in preventing spikelet degeneration under high-temperature stress has not been documented. Rice plants pretreated with SA (0–50 mmol L−1) were subjected to heat stress at 40 °C at the pollen mother cell meiosis stage for 10 days. The results indicated that there were no significant differences in grain yields and yield components among rice plants that were exogenously sprayed with SA (0–50 mmol L−1) under natural conditions. Under heat stress, the grain yield, spikelet number per panicle and setting rate in response to SA treatments were higher than under the control (0 mmol L−1 SA or NON-SA) treatment, especially with 1 and 10 mmol L−1 SA. A higher grain yield, spikelet number per panicle and setting rate were recorded in these two SA treatments compared with the NON-SA treatment. During this process, soluble sugars, proline, phytohormones including ABA, GA3, BRs, IAA, ZR and JA, and antioxidant enzymes, such as superoxide dismutase, peroxidase (POD), catalase (CAT) and ascorbate peroxidase (APX), were induced by SA. Moreover, soluble sugars, IAA, POD and APX in the spikelets with SA treatments were not only higher than with the NON-SA treatment but the changing patterns were also similar to that of the spikelet number per panicle under natural conditions and heat stress. Therefore, our results suggest that sugars, antioxidant enzymes and IAA might mediate SA to prevent spikelet degeneration caused by heat stress.



Similar content being viewed by others
References
Abdel-Wahed MSA, Amin AA, El-Rashad SM (2006) Physiological effect of some bioregulators on vegetative growth, yield and chemical constituents of yellow maize plants. World J Agric Sci 2:149–155
Aebi H (1983) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, Inc, New York, pp 273–288
Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M (2005) Cytokinin oxidase regulates rice grain production. Science 309:741–745
Barakat NA (2011) Oxidative stress markers and antioxidant potential of wheat treated with phytohormones under salinity stress. J Stress Physiol Biol 7:250–267
Barba-Espín G, Clemente-Moreno MJ, Alvarez S, García-Legaz MF, Hernandez JA, Díaz-Vivancos P (2011) Salicylic acid negatively affects the response to salt stress in pea plants. Plant Biol 13:909–917
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bonnecarrère V, Borsani O, Díaz P, Capdevielle F, Blanco P, Monza J (2011) Response to photoxidative stress induced by cold in japonica rice is genotype dependent. Plant Sci 180:726–732
Chao YY, Chen CY, Huang WD, Kao CH (2010) Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant Soil 329:327–337
Cingoz GS, Gurel E (2016) Effects of salicylic acid on thermotolerance and cardenolide accumulation under high temperature stress in Digitalis trojana Ivanina. Plant Physiol Biochem 105: 145–149
Dat JF, Lopez-Delgado H, Foyer CH, Scott IM (1998) Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol 116:1351–1357
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dong CJ, Wang XL, Shang QM (2011) Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Sci Hortic 129:629–636
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fageria NK (2007) Yield physiology of rice. J Plant Nutr 30:843–879
Fahad S, Bano A (2012) Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pak J Bot 44:1433–1438
Fariduddin Q, Hayat S, Ahmad A (2003) Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 41:281–284
Ferrante A, Savin R, Slafer GA (2013) Floret development and grain setting differences between modern durum wheats under contrasting nitrogen availability. J Exp Bot 64:169–184
Franklin KA, Lee SH, Patel D, Kumar SV, Spartz AK, Gu C, Wigge PA (2011) Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc Natl Acad Sci USA 108:20231–20235
Fu GF, Zhang CX, Yang XQ, Yang YJ, Chen TT, Zhao X, Fu WM, Feng BH, Zhang XF, Tao LX, Jin QY (2015) Action mechanism by which SA Alleviates high temperature-induced inhibition to spikelet differentiation. Chin J Rice Sci 29:637–647
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant physiol 59:309–314
Gray WM, del Pozo JC, Walker L, Hobbie L, Risseeuw E, Banks T, Estelle M (1999) Identification of an SCF ubiquitin–ligase complex required for auxin response in Arabidopsis thaliana. Gene Dev 13:1678–1691
Habibi G (2012) Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress. Acta Biol Szeged 56:57–63
Harfouche AL, Rugini E, Mencarelli F, Botondi R, Muleo R (2008) Salicylic acid induces H2O2 production and endochitinase gene expression but not ethylene biosynthesis in Castanea sativa in vitro model system. J Plant Physiol 165:734–744
Hayat, Shamsul B, Ahmad A (2007) Salicylic acid: biosynthesis, metabolism and physiological role in plants. Salicylic acid: a plant hormone. Springer, Netherlands, pp 1–14
Hayat S, Hasan SA, Fariduddin Q, Ahmad A (2008) Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J Plant Interact 3:297–304
He Y, Liu Y, Cao W, Huai M, Xu B, Huang B (2005) Effects of salicylic acid on heat tolerance associated with antioxidant metabolism in Kentucky bluegrass. Crop Sci 45:988–995
Huang C, Wang D, Sun L, Wei L (2016) Effects of exogenous salicylic acid on the physiological characteristics of Dendrobium officinale under chilling stress. Plant Growth Regul 79:199–208
IPCC (Intergovernmental panel on climate change) (2014) Mitigation of climate change. In: Edenhofer O, Pichs-Madruga R, Sokona Y, Farahani E, Kadner S, Seyboth K, Adler A, Baum I, Brunner S, Eickemeier P, Kriemann B, Savolainen J, Schloer S, von Stechow C, Zwickel T, Minx JC (ed) Climate change 2014: mitigation pathways and measures in the context of sustainable development. Contribution of working group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 1–26
Jiao Y, Wang Y, Xue D, Wang J, Yan M, Liu G, Qian Q (2010) Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat Genet 42:541–544
Kobayasi K, Yamane K, Imaki T (2001) Quantitative relation of spikelet degeneration with available carbohydrate in the mid-reproductive stage. Bull Fac Life Environ Sci Shimane Univ 6:23–29
Krantev A, Yordanova R, Janda T, Szalai G, Popova L (2008) Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165:920–931
LeClere S, Schmelz EA, Chourey PS (2010) Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels. Plant Physiol 153:306–318
Maehly A, Chance B (1954) Catalases and peroxidases. Methods Biochem Anal 1:357–424
Mishra BS, Singh M, Aggrawal P, Laxmi A (2009) Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PloS ONE 4:e4502
Miura K, Ikeda M, Matsubara A, Song XJ, Ito M, Asano K, Matsuoka M, Kitano H, Ashikari M (2010) OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat Genet 42:545–549
Nawrath C, Métraux JP (1999) Salicylic acid induction–deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11:1393–1404
Ookawa T, Hobo T, Yano M, Murata K, Ando T, Miura H, Ebitani T (2010) New approach for rice improvement using a pleiotropic QTL gene for lodging resistance and yield. Nat Commun 1:132
Patel R, Mohapatra PK (1992) Regulation of spikelet development in rice by hormones. J Exp Bot 43:257–262
Rajjou L, Belghazi M, Huguet R, Robin C, Moreau A, Job C, Job D (2006) Proteomic investigation of the effect of salicylic acid on Arabidopsis seed germination and establishment of early defense mechanisms. Plant Physiol 141:910–923
Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338
Ruan YL (2014) Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol 65:33–67
Satake T, Yoshida S (1978) High temperature induced sterility in indica rices at flowering. Jpn J Crop Sci 47:6–17
Satoh-Nagasawa N, Nagasawa N, Malcomber S, Sakai H, Jackson D (2006) A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 441:227–230
Shi Q, Bao Z, Zhu Z, Ying Q, Qian Q (2006) Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul 48:127–135
Slewinski TL (2011) Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: a physiological perspective. Mol Plant 4: 641–662
Smeekens S, Ma J, Hanson J, Rolland F (2010) Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13:273–278
Snider JL, Oosterhuis DM, Skulman BW, Kawakami EM (2009) Heat stress-induced limitations to reproductive success in Gossypium hirsutum. Physiol Plantarum 137:125–138
Tang RS, Zheng JC, Jin ZQ, Zhang DD, Huang YH, Chen LG (2008) Possible correlation between high temperature-induced floret sterility and endogenous levels of IAA, GAs and ABA in rice (Oryza sativa L.). Plant Growth Regul 54:37–43
Vanneste S, Friml J (2009) Auxin: a trigger for change in plant development. Cell 136:1005–1016
Vlot AC, Dempsey DMA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206
Wahid A, Close TJ (2007) Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol Plant 51:104–109
Wang Y, Zhang H, Hou P, Su X, Zhao P, Zhao H, Liu S (2014) Foliar-applied salicylic acid alleviates heat and high light stress induced photoinhibition in wheat (Triticum aestivum) during the grain filling stage by modulating the psbA gene transcription and antioxidant defense. Plant Growth Regul 73:289–297
Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414:562–565
Xie Z, Zhang ZL, Hanzlik S, Cook E, Shen QJ (2007) Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid inducible WRKY gene. Plant Mol Biol 64:293–303
Yang Y, Qi M, Mei C (2004) Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J 40:909–919
Yao Y, Yamamoto Y, Yoshida T, Nitta Y, Miyazaki A (2000) Response of differentiated and degenerated spikelets to top-dressing, shading and day/night temperature treatments in rice cultivars with large panicles. Soil Sci Plant Nutr 46:631–641
Yokoyama C, TsudaM, Hirai Y (2002) Effects of plant growth regulators on number of spikelets per panicle in rice (Oryza sativa L.) under saline flooding conditions. Jpn J. Crop Sci 71:376–382
Yoshida A, Ohmori Y, KitanoH, Taguchi-Shiobara F, Hirano HY (2012) ABERRANT SPIKELET AND PANICLE1, encoding a TOPLESS-related transcriptional co-repressor, is involved in the regulation of meristem fate in rice. Plant J 70:327–339
Yoshida A, Sasao M, Yasuno N, Takagi K, Daimon Y, Chen R, Nagamura Y (2013) TAWAWA1, a regulator of rice inflorescence architecture, functions through the suppression of meristem phase transition. P Natl Acad Sci USA 110:767–772
Yusuf M, Hasan SA, Ali B, Hayat S, Fariduddin Q, Ahmad A (2008) Effect of salicylic acid on salinity-induced changes in Brassica juncea. J Integr Plant Biol 50:1096–1102
Zhang D, Yuan Z (2014) Molecular control of grass inflorescence development. Annu Rev Plant Biol 65:553–578
Zhang CX, Fu GF, Yang XQ, Yang YJ, Zhao X, Chen TT, Tao LX (2016) Heat stress effects are stronger on spikelets than on flag leaves in rice due to differences in dissipation capacity. J Agron Crop Sci 202:394–408
Acknowledgements
This work was funded by the National Natural Science Foundation of China (Grant No. 31501264 and 31561143003), the China National Rice Research Institute (Grant No. 2014RG004-4), the Special Fund for Agro-Scientific Research in The Public Interest (Grant No. 201203029), and the National System of Rice Industry (Grant No. CARS-01-27); National Food Science and Technology Project (2016 YFD 0300208).
Author information
Authors and Affiliations
Corresponding authors
Additional information
C. X. Zhang and B. H. Feng have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, C.X., Feng, B.H., Chen, T.T. et al. Sugars, antioxidant enzymes and IAA mediate salicylic acid to prevent rice spikelet degeneration caused by heat stress. Plant Growth Regul 83, 313–323 (2017). https://doi.org/10.1007/s10725-017-0296-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-017-0296-x