Abstract
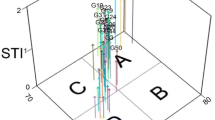
The present investigation was carried out to characterize genotypic variability in chickpea for water deficit tolerance by exploring the antioxidative defense system and seedling growth. Twenty nine chickpea genotypes including cultivars and advanced lines were grown under control and water deficit conditions induced by adding 3 % mannitol. The genotypes showed differential response in seedling growth under water deficit conditions. The activities of catalase (CAT) and superoxide dismutase (SOD) were observed to be differentially expressed in the roots of various genotypes, under control and water deficit conditions. The contents of H2O2, malondialdehyde (MDA) and proline were also observed to be variable in the roots of all the genotypes, under control and water deficit conditions. Stress tolerance index for the various parameters, viz, CAT and SOD activity, H2O2, MDA and proline content, root length, shoot length and their biomass was determined and the level of stress resistance calculated. The genotypes which showed increased activities of CAT and SOD, decreased contents of H2O2 and MDA together with least affected seedling growth under water deficit conditions exhibited higher stress resistance capacity. Multivariate principal component analysis for all the parameters affected under water deficit conditions, grouped the genotypes into three clusters having different (high, moderate and low) levels of stress resistance. Complete linkage clustering grouped these genotypes into two major clusters-I and II. The genotypes present in sub–sub cluster ‘A1’ and sub cluster ‘B’ of major cluster-I have been observed to possess high stress resistance levels for respective parameters. It can thus be concluded that chickpea genotypes exhibiting increased stress resistance levels in relation to SOD and CAT activities, H2O2 and MDA contents and seedling growth would have higher stress tolerance under water deficit conditions.





Similar content being viewed by others
References
Anjum SA, Wang LC, Farooq M, Hussain M, Xue LL, Zou CM (2011) Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J Agron Crop Sci 197:177–185
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Critical Rev Plant Sci 24:23–58
Bates LS (1973) Rapid determination of free proline content for water stress studies. Plant Soil 39:205–207
Boo YC, Jung Y (1999) Water deficit induced oxidative stress and antioxidative defenses in rice plants. Plant Physiol 155:255–261
Brou YC, Zeze A, Diouf O, Eyletters M (2007) Water stress induces overexpression of superoxide dismutases that contribute to the protection of cowpea plants against oxidative stress. Afr J Biotech 6:1982–1986
Chance B, Maehley AC (1955) Assay of catalases and peroxidases. Meth Enzymol 2:764–775
Chugh V, Gupta AK, Grewal MS, Kaur N (2012) Response of antioxidative and ethanolic fermentation enzymes in maize seedlings of tolerant and sensitive genotypes under short term waterlogging. Indian J Exp Biol 50:577–582
Dat JF, Inze D, Van Breusegem F (2001) Catalase deficient tobacoo plants: tools for in planta studies on the role of hydrogen peroxide. Redox Rep 6:37–42
Davey MW, Stals E, Pains B, Keulemans J, Swennen RL (2005) High throughput determination of malondialdehyde in plant tissues. Anal Biochem 347:201–207
Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plants in drying soil. Annual Rev Plant Physiol Plant Mol Biol 42:55–76
Devi R, Kaur N, Gupta AK (2012) Potential of antioxidant enzymes in depicting drought tolerance of wheat (Triticum aestivum L.). Indian J Biochem Biophys 49:257–265
Esterbaurer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malondialdehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Everitt BS, Landau S, Leese M (2001) Cluster analysis, 4th edn. Edward Arnold, London
Eyidogan F, Oz MT (2007) Effect of salinity on antioxidant responses of chickpea seedlings. Acta Physiol Plant 29:485–493
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sust Dev 29:185–212
Feierabend J, Dehne S (1996) Fate of the porphyrin cofactors during the light dependent turnover of catalase and of the photosystem II reaction centre protein D1 in mature rye leaves. Planta 198:413–422
Fernandez GCJ (1992) Effective selection criteria for assessing plant stress tolerance. In: Proceedings of symposium. Taiwan, 13–16 Aug. Chap 25, pp 257–270
Food and Agriculture Organization (2007) FAOSTAT. The food and agricultural organization of the united nations, Rome. http://www.faostat.fao.org/site/567/default.aspx
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Ann Rev Plant Physiol Plant Mol Biol 51:463–499
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Islam MM, Hoque MA, Okuma E, Banu MNA, Shimoishi Y, Nakamura Y, Murata Y (2009) Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol 166:1587–1597
Jaleel CA, Gopi R, Sankar B, Manivannan P, Kishorekhyumar A, Sridharan R, Panneerselvam R (2007) Studies on germination, seedling vigour, lipid peroxidation and proline metabolism in Catharanthus roseus seedlings under salt stress. South Afr J Bot 73:190–195
Jayashree B, Buhariwalla HK, Shinde S, Crouch JH (2005) A legume genomics resource: the chickpea root expressed sequence tag database. Elec J Biotech 8(2):128–133
Kaur H, Gupta AK, Kaur N, Sandhu JS (2009) Differential response of the antioxidant system in wild and cultivated genotypes of chickpea. Plant Growth Regul 57:109–114
Kaur S, Arora M, Gupta AK, Kaur N (2012) Exploration of biochemical and morphological diversity in chickpea seeds to categorize cold stress-tolerant and susceptible genotypes. Acta Physiol Plant 34:569–580
Khedr AH, Abbas MA, Wahid AA, Quick WP, Abogadallah GM (2003) Proline induces the expression of salt-stress responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J Exp Bot 54:2553–2562
Kumar J, Abbo S (2001) Genetics of flowering time in chickpea and its bearing on productivity in semiarid environments. Adv Agron 72:107–138
Lei YB, Yin CY, Li CY (2006) Differences in some morphological, physiological and biochemical responses to drought stress in two contrasting populations of Populus Przewalskii. Physiol Plant 127:182–191
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Mafakheri A, Siosimardeh A, Bahramnejad B, Struik PC, Sohrabi E (2010) Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust J Crop Sci 4(8):580–585
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the auto-oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:169–174
Matysik J, Alia BB, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Moller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Munne-Bosch S, Penvelas J (2003) Photo and antioxidative protection and a role for salicylic acid during drought and recovery in field grown Phillyrea angustifolia plants. Planta 217:758–766
Patel PK, Hemantaranjan A (2012) Antioxidant defense system in chickpea (Cicer arietinum L.): influence by drought stress implemented at pre- and post-anthesis stage. Am J Plant Physiol. doi:10.3932/ajpp.2012
Rohlf FJ (1998) NTSYS-pc.version 2.0. Exeter Software. Setauket, NY
Sairam RK, Saxena DC (2000) Oxidative stress and antioxidants in wheat genotypes: possible mechanism of water stress tolerance. J Agron Crop Sci 184:55–61
Sairam RK, Deshmukh PS, Saxena DC (1998) Role of antioxidant systems in wheat genotypes tolerant to water stress. Biol Plant 41:387–394
Scandalios JG (1997) Molecular genetics of superoxide dismutases in plants. In: Scandalios JG (ed) Oxidative stress and the molecular biology of antioxidative defenses. Plainview, Cold spring harbor, pp 527–568
Shao HB, Liang ZS, Shao MA (2005) Changes of antioxidative enzymes and MDA content under soil water deficits among 10 wheat (Triticum aestivum L.) genotypes at maturation stage. Colloids surf Biointerfaces 45:7–13
Singh S, Gupta AK, Kaur N (2012) Differential responses of antioxidative defense system to long term field drought in wheat (Triticum aestivum L.) genotypes differing in drought tolerance. J Agron Crop Sci 198:185–195
Sinha AK (1971) Colorimetric assay of catalase. Anal Biochem 47:389–394
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends in Plant Sci 15:89–97
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759
Willekens H, Chamnogpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inze D, Van Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defense in C3 plants. EMBO J 16:4806–4816
Yang Y, Han C, Liu Q, Lin B, Wang J (2008) Effect of drought and low light on growth and enzymatic antioxidant system of picea asperata seedlings. Acta Physiol Plant 30:433–440
Zaefyzadeh M, Quliyev RA, Babayeva SM, Abbasov MA (2009) The effect of the interaction between genotypes and drought stress on the superoxide dismutase and chlorophyll content in durum wheat landraces. Turk J Biol 33:1–7
Acknowledgments
Thanks are due to Council of Scientific and Industrial Research, New Delhi for funding this project under the Emeritus Scientist scheme to Narinder Kaur.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, K., Kaur, N., Gupta, A.K. et al. Exploration of the antioxidative defense system to characterize chickpea genotypes showing differential response towards water deficit conditions. Plant Growth Regul 70, 49–60 (2013). https://doi.org/10.1007/s10725-012-9777-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9777-0