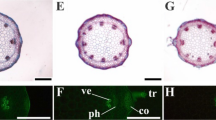
Abstract
Plants are able to dynamically adapt to their environment by reprogramming of their growth and development. Copper (Cu2+) excess modifies shoot and root architecture of plants by a lesser known mechanism, therefore the involvement of a major hormone component (auxin) and a signal molecule (nitric oxide) in Cu2+-induced morphological responses were studied in Arabidopsis using microscopic methods. Auxin-inducible gene expression was visualized in DR5::GUS Arabidopsis and nitric oxide (NO) levels were detected by DAF-FM fluorophore in the stem and root system. Copper excess caused the inhibition of stem and root growth of Arabidopsis, during which cell elongation, division and expansion were also affected. The symptoms of stress-induced morphogenic response were found in the root system of 25 μM Cu2+-treated plants. In both organs, the decrease of auxin-dependent gene expression was found, which can partly explain the growth inhibitions. Besides hormonal system, nitric oxide metabolism was also affected by Cu2+. In root tips, copper excess induced NO generation, while NO content in lateral roots was not affected by the treatments. Using nia1nia2 mutants, nitrate reductase enzyme as a putative source of Cu2+-induced NO was identified in Arabidopsis primary roots.







Similar content being viewed by others
References
Alaoui-Sossé B, Genet P, Vinit-Dunand F, Toussaint M-L, Epron D, Badot P-M (2004) Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci 166:1213–1218
Aloni R, Schwalm K, Langhans M, Ullrich CI (2002) Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 216:841–853
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905
Fernández-Marcos M, Sanz L, Lewis DR, Muday GK, Lorenzo O (2011) Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. PNAS 108:18506–18511
Foresi N, Correa-Aragunde N, Parisi G, Calo G, Salerno G, Lamattina L (2010) Characterization of a nitric oxide synthase from the plant kingdom: NO generation from the green alga Ostreococcus tauri is light irradiance and growth phase dependent. Plant Cell 22:3816–3830
Halliwell B, Gutteridge JMC (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219:1–14
He Y, Tang R-H, Hao Y, Stevens RD, Cook CW, Ahn SM, Jing L, Yang Z, Chen L, Gio F, Fiorani F, Jackson RB, Crawford NM, Pei Z-M (2004) Nitric oxide represses the Arabidopsis floral transition. Science 305:1968–1971
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jiang W, Liu D, Li H (2000) Effects of Cu2+ on root growth, cell division, and nucleolus of Helianthus annuus L. Sci Total Environ 256:59–65
Kolbert Zs, Bartha B, Erdei L (2008) Osmotic stress- and indole-3-butyric acid-induced NO generations are partially distinct processes in root growth and development in Pisum sativum L. Physiol Plant 133:406–416
Kolbert Zs, Ortega L, Erdei L (2010) Involvement of nitrate reductase (NR) in osmotic stress-induced NO generation of Arabidopsis thaliana L. roots. J Plant Physiol 167:77–80
Kollmeier M, Felle HH, Horst WJ (2000) Genotypical differences in aluminium resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminium? Plant Physiol 122:945–956
Lequeux H, Hermans C, Lutts S, Verbruggen N (2010) Response to copper excess in Arabidopsis thaliana: impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol Biochem 48:673–682
Lombardo MC, Graziano M, Polacco J, Lamattina L (2006) Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav 1:28–33
Maksymiec W, Baszyński T (1998) The effect of Ca2+ on photosynthetic activity and assimilate distribution in Cu2+ stressed bean plants. In: Garab G (ed) Photosynthesis: mechanisms and effects. Kluwer, Dordrecht, pp 2669–2672
Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124:33–44
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Oliveira HC, Saviani EE, Oliveira JFP, Salgado I (2010) Nitrate reductase-dependent nitric oxide synthesis in the defence response of Arabidopsis thaliana against Pseudomonas syringae. Trop Plant Path 35:104–107
Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129:954–956
Pasternak T, Rudas V, Potters G, Jansen MAK (2005) Morphogenic effects of abiotic stress: reorientation of growth in Arabidopsis thaliana seedlings. Environ Exp Bot 53:299–314
Pető A, Lehotai N, Lozano-Juste J, León J, Tari I, Erdei L, Kolbert Zs (2011) Involvement of nitric oxide (NO) in signal transduction of copper-induced morphological responses in Arabidopsis seedlings. Ann Bot 108:449–457
Pilon-Smits E, Pilon M (2002) Phytoremediation of metals using transgenic plants. Crit Rev Plant Sci 21:439–456
Potters G, Pasternak TP, Guisez Y, Jansen MAK (2009) Different stresses, similar morphogenetic responses: integrating a plethora of pathways. Plant, Cell Environ 32:158–169
Shi H-T, Li R-J, Cai W, Liu W, Wang L-W, Lu Y-T (2012) Increasing nitric oxide content in Arabidopsis thaliana by expressing rat neuronal nitric oxide synthase resulted in enhanced stress tolerance. Plant Cell Physiol 53:344–357
Wang Y, Li K, Li X (2009) Auxin redistribution modulates plastic development of root system architecture under salt stress in Arabidopsis thaliana. J Plant Physiol 166:1637–1645
Wang H-H, Huang J-J, Bi Y-R (2010) Nitrate reductase-dependent nitric oxide production is involved in aluminium tolerance in red kidney bean roots. Plant Sci 179:281–288
Wilkinson JQ, Crawford NM (1993) Identification and characterization of a chlorate resistant mutant of Arabidopsis with mutations in both NIA1 and NIA2 nitrate reductase structural genes. Mol Gen Genet 239:289–297
Xiong J, Fu G, Tao L, Zhu C (2010) Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch Biochem Biophys 497:13–20
Xu YC, Zhao BL (2003) The main origin of endogenous NO in higher non-leguminous plants. Plant Physiol Biochem 41:833–838
Xu L, Yin H, Liu X, Li X (2009) Salt affects plant Cd-stress responses by modulating growth and Cd accumulation. Planta 231:449–459
Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36:409–430
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61:49–64
Zhao C-R, Ikka T, Sawaki Y, Kobayashi Y, Suzuki Y, Hibino T, Sato S, Sakurai N, Shibata D, Koyama H (2009) Comparative transcriptomic characterization of aluminium, sodium chloride, cadmium and copper rhizotoxicities in Arabidopsis thaliana. BMC Plant Biol 9:32
Zolla G, Heimer YM, Barak S (2010) Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. J Exp Bot 61:211–224
Acknowledgements
The NR double mutant nia1,nia2 seeds were kindly provided by Prof. Dr. F. E. Scherer (Universität Hannover, Germany). This work was supported by the Hungarian Scientific Research Fund Grant no. OTKA PD100504. The publication was supported by the European Union and co-funded by the European Social Fund (project number: TÁMOP-4.2.2/B-10/1-2010-0012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Zsuzsanna Kolbert and Andrea Pető contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kolbert, Z., Pető, A., Lehotai, N. et al. Long-term copper (Cu2+) exposure impacts on auxin, nitric oxide (NO) metabolism and morphology of Arabidopsis thaliana L.. Plant Growth Regul 68, 151–159 (2012). https://doi.org/10.1007/s10725-012-9701-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9701-7