Abstract
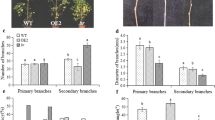
Bast fibre development in jute (Corchorus spp.) is a complex process that involves the differentiation of secondary phloic fibres (SPF) from the cambium followed by lignification of the fibre wall. We have identified a unique radiation-induced bast fibre-shy mutant of dark jute (C. olitorius L.), which is concurrently defective in the differentiation of SPF and secondary xylem (wood) but develops lignified fibre cells. It displays the most unusual phenotype with stunted growth and abnormal leaf shape, matures earlier, yields significantly less bast fibres and wood, and produces poorer quality fibres than its parental wild-type. Cambial activities in the mutant and the normal type were monitored by estimating the fibre content that entails the total number of fibre cell bundles (FCBs) in an entire transversal section. The results show that a multi-fold reduction of bast fibre yield in the mutant is related to development-specific loss of cambium function along the length of the stem from to top to bottom. Since lignification of the fibre wall in the mutant is not only normal but also developmentally uniform, cambium function may be unrelated to the lignification process during bast fibre development. Lignin does not influence bast fibre strength and fineness. The architecture of the mostly triangular FCB wedges, which is governed by a balanced growth between radially elongating FCBs and tangentially expanding ray cells due to development-specific activation of the fusiform and ray initials of the cambium, conditions fibre fineness. Our study shows that mutation could specifically impair the cambial activity by rendering those initials that differentiate the SPF and secondary xylem nonfunctional.





Similar content being viewed by others
Abbreviations
- bfs :
-
Bast fibre-shy
- EDTA:
-
Ethylenediaminetetraacetic acid
- FCB:
-
Fibre cell bundle
- G:
-
Guaiacyl unit
- OMU:
-
Corchorus olitorius mutant
- PPF:
-
Primary phloic fibre
- S:
-
Syringyl unit
- SDS:
-
Sodium dodecyl sulfate
- SPF:
-
Secondary phloic fibre
References
Aloni R (1988) Vascular differentiation within the plant. In: Roberts LW, Gahan PB, Aloni R (eds) Vascular differentiation and plant growth regulators. Springer-Verlag, Berlin, pp 39–62
Aloni R, Tollier MT, Monties B (1990) The role of auxin and gibberellin in controlling lignin formation in primary phloem fibres and in xylem of Coleus blumei stems. Plant Physiol 94:1743–1747
Anonymous (2008) Determination of acid detergent fibre (ADF) and acid detergent lignin (ADL) in feed and forage using FibreCap™ 2021/2023 in agreement with EN ISO 13906:2008. Application note 3804. FOSS Analytical A/S, Hilleroed, p 10
Bandopadhyay SB, Mukhopadhyay SK (1964) Assessment of jute fibre bundle strength. Jute Bull 27:193–199
Clifford MN (1974) Specificity of acidic phloroglucinol reagents. J Chromatogr 94:321–324
Dashek WV (1997) Methods in plant biochemistry and molecular biology. CRC Press, Boca Raton
Day A, Ruel K, Neutelings G, Crônier D, David H, Hawkins S, Chabbert B (2005) Lignification in the flax stem: evidence for an unusual lignin in bast fibres. Planta 222:234–245
Del Rio JC, Rencoret J, Matques G, Li J, Gellerstedt G, Jiménez-Barbero J, Martínez AT, Gutiérrez A (2009) Structural characterization of the lignin from jute (Corchorus capsularis) fibers. J Agric Food Chem 57:10271–10281
Esau K (1953) Plant Anatomy. John Wiley & Sons, Inc., New York
Evert RF, Esau K (2006) Esau’s plant anatomy: meristems, cells, and tissues of the plant body: their structure, function and development. John Wiley and Sons, New York
Fahn A (1990) Plant anatomy, 4th edn. Pergamon Press, Oxford
Hazra SK, Karmakar PG (2008) Anatomical parameters of bast fibres for yield and quality improvement. In: Karmakar PG, Hazra SK, Ramasubramanian T, Mandal RK, Sinha MK, Sen HS (eds) Jute and allied fibre updates. Central Research Institute for Jute and Allied Fibres, Kolkata, pp 38–45
Heywood VH, Brummitt RK, Culham A, Seberg O (2007) Flowering plant families of the world. Royal Botanical Gardens, Kew
Iiyama K, Pant R (1988) The mechanism of the Maüle color reaction. Introduction of methylated syringyl nuclei into soft-wood lignin. Wood Sci Technol 22:167–175
Islam AS, Sarkanen K (1993) The isolation and characterization of lignins of jute (Corchorus capsularis). Holzforschung 47:123–132
Jahan MS, Chowdhury DAN, Islam MK, Moeiz SMI (2007) Characterization of lignin isolated from some nonwood available in Bangladesh. Bioresour Technol 98:465–469
Joshua DC, Rao NS, Gottschalk W (1972) Evolution of leaf shape in jute. Indian J Genet Plant Breed 32:392–398
Karmakar PG, Hazra SK, Sinha MK, Chaudhury SK (2008) Breeding for quantitative traits and varietal development in jute and allied fibre crops. In: Karmakar PG, Hazra SK, Ramasubramanian T, Mandal RK, Sinha MK, Sen HS (eds) Jute and allied fibre updates: production and technology. Central Research Institute for Jute and Allied Fibres, Kolkata, pp 57–75
Kundu BC (1944) Anatomy of jute stem with special reference to cambial activity and distribution of fibres in relation to leaf-trace system. J Royal Asiatic Soc Bengal (Sci) 10:27–52
Kundu BC (1956) Jute: world’s foremost bast fibre. I. Botany, agronomy, diseases and pests. Econ Bot 10:103–133
Kundu BC, Basak KC, Sarkar PB (1959) Jute in India. The Indian Central Jute Committee, Calcutta
Lee E-K, Jin Y-W, Park JH, Yoo YM, Hong SM, Amir R, Yan Z, Kwon E, Elfick A, Tomlinson S, Halbritter F, Waibel T, Yun B-W, Loake GJ (2010) Cultured cambial meristematic cells as a source of plant natural products. Nature Biotechnol 28:1213–1217
Lewis NG, Yamamoto E (1990) Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol 41:455–496
Lux A, Morita S, Abe J, Ito K (2005) An improved method for clearing and staining free-hand sections and whole-mount samples. Ann Bot 96:989–996
Mahapatra AK, Saha A, Gupta D (2006) Catalogue on evaluation of tossa jute germplasm (Corchorus olitorius L.). Central Research Institute for Jute and Allied Fibres Kolkata, p 41
Maiti RK (1979) A study of the microscopic structure of the fibre strands of common Indian bast fibres and its economic implications. Econ Bot 33:78–87
Maiti RK (1980) Plant fibres. Bishen Singh Mahendra Pal Singh, Dehra Dun
Maiti RK (1997) World fibre crops. Oxford and IBH Publishing Company, New Delhi
Maiti RK, Mitra GC (1972) Cambial activity in relation to the production of fibre bundles at different phases of growth in white jute. Bull Bot Soc Bengal 26:79–85
Maiti RK, Satya P (2010) Fibre bundle anatomy determines the yield potential of bast fibre (long fibre): a hypothesis. Environ Biotechnol 2:A1–A6
Maiti RK, Rodriguez HG, Satya P (2011) Horizon of world plant fibres: an insight. Pushpa Publishing House, Kolkata
Majumdar S (2002) Prediction of fibre quality from anatomical studies of jute stem: part I- prediction of fineness. Indian J Fibre Textile Res 27:248–253
Meshitsuka G, Nakano J (1979) Studies on the mechanism of lignin color reaction (XIII): Maüle color reaction (9). Mokuzai Gakkaishi 25:588–594
Mitra GC (1984) Genetic control of secondary phloic fibres in jute (Corchorus capsularis L.). Genetica 63:9–11
Mitra GC, Maiti RK (1974) Cambial activity in relation to yield potential in ‘Tossa’ jute (C. olitorius). Bull Bot Soc Bengal 28:35–39
Palit P (1999) Jute. In: Smith DL, Hamel C (eds) Crop yield, physiology and processes. Springer-Verlag, Berlin, pp 271–286
Palit P, Meshram JH (2004) Physiological characterization of a phenotypically distinct jute (Corchorus olitoius) genotype. Plant Genet Resour 2:175–180
Palit P, Meshram JH (2008) Physiology of jute yield and quality. In: Karmakar PG, Hazra SK, Ramasubramanian T, Mandal RK, Sinha MK, Sen HS (eds) Jute and allied fibre updates. Central Research Institute for Jute and Allied Fibres, Kolkata, pp 112–124
Palit P, Sengupta G, Datta P, Meshram JH (2001) Lignin and lignification with special reference to its down regulation for the improvement of wood and bast fibre quality. Indian J Plant Physiol 6(NS):217–228
Palit D, Meshram JH, Palit P (2006a) Biology of jute fibre quality. Sci Cult 72:379–382
Palit D, Meshram JH, Palit P (2006b) Genotypic variation in the characteristics of secondary phloem fibre cells of jute in relation to its yield and quality. J Bot Soc Bengal 60:32–37
Ross JJ (1994) Recent advances in the study of gibberellin mutants. Plant Growth Regul 15:193–206
Rowell RM, Stout HP (2007) Jute and kenaf. In: Lewin M (ed) Handbook of fibre chemistry, 3rd edn. CRC Press, Boca Raton, pp 405–452
Sarkar D, Sinha MK, Kundu A, Kar CS, Saha A, Kharbikar LL, Mahapatra BS (2010) Why is ramie the strongest yet stiffest of bast fibres? Curr Sci 98:1570–1572
Satya P, Mahapatra AK, Maiti RK (2011) Fibre anatomy structure: a good predictor for fibre yield and fibre quality in Corchorus capsularis. Int J Bioresour Stress Manage 2:263–267
Sengupta G, Palit P (2004) Characterization of a lignified secondary phloem fibre-deficient mutant of jute (Corchorus capsularis). Ann Bot 93:211–220
Sinha NG, Bandopadhyay SB (1968) An air-flow method for the determination of the fibre fineness of jute and mesta. J Text Inst 59:148–156
Summerscales J, Dissanayake NPJ, Virk AS, Hall W (2010) A review of bast fibres and their composites. Part 1- Fibres as reinforcements. Composites Part A 41:1329–1335
Thakare RG, Joshua DC, Rao NS (1973) Induced viable mutations in Corchorus olitorius L. Indian J Genet Plant Breed 33:204–228
Turley RB (2002) Registration of MD17 fibreless upland cotton as a genetic stock. Crop Sci 42:994–995
Acknowledgments
Comments and suggestions on the manuscript from an anonymous reviewer and the Editor are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kundu, A., Sarkar, D., Mandal, N.A. et al. A secondary phloic (bast) fibre-shy (bfs) mutant of dark jute (Corchorus olitorius L.) develops lignified fibre cells but is defective in cambial activity. Plant Growth Regul 67, 45–55 (2012). https://doi.org/10.1007/s10725-012-9660-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9660-z