Abstract
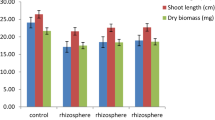
The present study was conducted to investigate the effect of the residue of Chenopodium murale L. on growth, nodulation and macromolecule content of two legume crops, viz., Cicer arietinum L. (chickpea) and Pisum sativum L. (pea). A significant reduction in root and shoot length as well as dry matter accumulation occurred when both the legumes were grown in the soil amended with 5, 10, 20 and 40 g residue kg−1 soil. In general, a gradual decline in growth was associated with an increasing amount of residues in the soil. There was also a significant reduction in total chlorophyll content and the amounts of protein and carbohydrates (macromolecules) in plants growing in the residue-amended soil. The nodulation was completely absent in chickpea and pea when the plants were grown in the soil amended with 10 and 20 g residue kg−1 soil, respectively. At a lower rate of residue amendment (5 g kg−1 soil), a significant decline in nodule number and weight, and leghaemoglobin content was recorded. Root oxidizability, an indirect measure of tissue viability and cellular respiration, was adversely affected in both the legumes under various treatments of residue amendment. The observed growth reduction concomitant with increased proline accumulation indicated the presence of some inhibitory compounds in the residue-amended soil. It was rich in phenolics identified as protocatechuic, ferulic, p-coumaric and syringic acid with 12.8, 30.4, 20.2 and 33.6% relative content, respectively. The results suggest that the residue of C. murale releases phenolic allelochemicals, which deleteriously affect the growth, nodulation and macromolecule content of chickpea and pea.


Similar content being viewed by others
References
Anonymous (1992) Chenopodium murale. In: Phondke GP (ed) The wealth of India – raw materials, revised series, vol III (Ca–Ci). Council of Scientific and Industrial Research, New Delhi, p 471
Appel HM (1993) Phenolics in ecological interactions: the importance of oxidation. J Chem Ecol 19:1521–1552
Arnon DI (1949) Copper enzymes in isolated chloroplasts: Polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Bais HP, Park S-W, Weir TL, Callaway RM, Vivanco JM (2004) How plants communicate using the underground information superhighway. Trends Plant Sci 9:26–32
Bates LS, Walderen RD, Taere ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Batish DR, Singh HP, Pandher JK, Arora V, Kohli RK (2002) Phytotoxic effect of Parthenium residues on the growth of radish and chickpea and selected soil properties. Weed Biol Manage 2:73–78
Batish DR, Singh HP, Kaur S, Kohli RK (2006) Phytotoxicity of Ageratum conyzoides towards growth and nodulation of Cicer arietinum. Agr Ecosyst Environ 113:399–401
Batish DR, Lavanya K, Singh HP, Kohli RK (2007) Root-mediated allelopathic interference of Nettle-leaved Goosefoot (Chenopodium murale) on wheat (Triticum aestivum). J Agron Crop Sci (in press), doi: 10.1111/j.1439-037X.2006.00243
Bergmark CL, Jackson WA, Volk RJ, Blum U (1992) Differential inhibition by ferulic acid of nitrate and ammonium uptake in Zea mays L. Plant Physiol 98:639–645
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83
Blum U (1998) Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on allelopathic interactions. J Chem Ecol 24:685–708
Blum U, Shafer SR (1988) Microbial populations and phenolic acids in soil. Soil Biol Biochem 20:793–800
Blum U, Shafer SR, Lehman ME (1999) Evidence for inhibitory allelopathic interactions involving phenolic acids in field soils: concepts vs. an experimental model. Crit Rev Plant Sci 18:673–693
Castells E, Penuelas J, Valentine DW (2005) Effects of plant leachates from four boreal understorey species on soil N mineralization, and white Spruce (Picea glauca) germination and seedling growth. Ann Bot 95:1247–1252
Comas LH, Eissenstat DM, Lasko AN (2000) Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytol 147:171–178
Datta SC, Ghosh KN (1982) Effect of pre-sowing treatment of mustard seeds with leaf and inflorescence extracts of Chenopodium murale. Indian J Weed Sci 14:1–6
El-Khatib AA, Hegazy AK, Galal HK (2003) Allelopathy in the rhizosphere and amended soil of Chenopodium murale. Weed Biol Manage 41:37–45
Flores A, Grau A, Laurich F, Dorffling K (1988) Effects of new terpenoid analogues of abscissic acid on chilling and freezing resistances. J Plant Physiol 132:362–363
Guertin P (2003) USGS weeds in the west project: status of introduced plants in the southern Arizona parks—fact sheet for: Chenopodium murale L. US Geological Survey, Southwest Biological Science Center, Sonoran Desert Field Station, University of Arizona, Arizona, USA. Available via DIALOG. http://sdrsnet.srnr.arizona.edu/data/sdrs/ww/docs/chenmura.pdf. Cited 31 Dec 2003
Halsall DM, Leigh JH, Gollasch, Holgate M (1995) The role of Allelopathy in legume decline in pastures. II. Comparative effects of pasture, crops and weed residues on germination, nodulation and root growth. Aust J Agr Res 46:189–207
Hiscox TD, Israelstam GF (1979) A method for extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Holm L, Doll J, Holm E, Pancho J, Herberger J (1997) World weeds: natural histories and distribution. John Wiley & Sons, New York
Klein K, Blum U (1990) Effects of soil nitrogen level on ferulic acid inhibition of cucumber leaf expansion. J Chem Ecol 16:1371–1383
Kohli RK, Batish DR, Singh HP (1998) Allelopathy and its implications in agroecosystems. J Crop Prod 1:169–202
Loewus FA (1952) Improvement in anthrone method for determination of carbohydrates. Anal Chem 24:219
Lowry OH, Rosenborough NJ, Farr AL, Rendall RJ (1951) Protein estimation with folin-phenol reagent. J Biol Chem 193:265–275
Mallik MAB (1999) Allelopathy and nitrogen fixation in legumes. In: Narwal SS (ed) Allelopathy update: basic and applied aspects, vol 2. Oxford and IBH Publishing Company Private Limited, New Delhi, pp 177–200
Mallik MAB, Tesfai K (1988) Allelopathic effect of common weeds on soybean growth and soybean-Bradyrhizobium symbiosis. Plant Soil 112:177–182
Marshall RM, Anderson S, Batcher M, Comer P, Cornelius S, Cox R, Gondor A, Gori D, Humke J, Aquilar RP, Paredes Parra IE, Schwartz S (2000) An ecological analysis of conservation priorities in the Sonoran Desert Ecoregion. Nature Conservancy—Arizona Chapter, Sonoran Institute, Instituto del Medio Ambiente y el Desarrollo Sutentable del Estado de Sonora—Mexico, and University of Arizona, Arizona, USA
Mizutani J (1999) Selected allelochemicals. Crit Rev Plant Sci 18:653–671
Prati D, Bossdorf O (2004) Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). Am J Bot 91:285–288
Qasem JR (1995) Allelopathic effect of Amaranthus retroflexus and Chenopodium murale on vegetable crops. Allelopathy J 2:49–66
Qasem JR, Foy CL (2001) Weed Allelopathy, its ecological impacts and future prospects: a review. J Crop Prod 4:43–119
Rani D, Kohli RK (1991) Fresh matter is not an appropriate unit for chlorophyll content: experiences from experiments on effects of herbicides and allelopathic substances. Photosynthetica 25:655–658
Rice EL (1984) Allelopathy. Academic Press, Orlando
Rice EL (1995) Biological control of weeds and plant diseases—Advances in applied allelopathy. University of Oklahoma Press, Norman
Rice EL, Pancholy SK (1973) Inhibition of nitrification by climax ecosystems. II. Additional evidence and possible role of tannins. Am J Bot 60:691–702
Shen HC, Zhou WJ, Xi HF, Ye QF (1991) A preliminary study of physiological and yield effects of paclobutrazol on Brassica napus. Acta Agr Univ Zhejiang 17:423–426
Singh HP, Kohli RK, Batish DR (2001) Allelopathy in agroecosystems: an overview. J Crop Prod 4:1–41
Swain T, Hillis WE (1959) The phenolic constituents of Prunus domestica I. The quantitative analysis of constituents. J Sci Food Agr 10:63–68
Wardle DA, Nicholson KS, Ahmed KS, Rahman A (1994) Interference effects of the invasive plant Carduus nutans L. against the nitrogen fixation ability of Trifolium repens L. Plant Soil 163:287–297
Weston LA, Putnam AR (1985) Inhibition of growth, nodulation and nitrogen fixation of legumes by quackgrass. Crop Sci 25:561–565
Wilson DO, Reisenauer HM (1963) Determination of leghemoglobin in legume nodule. Anal Biochem 6:27–30
Xuan TD, Tawata S, Khanh TD, Chung IM (2005) Decomposition of allelopathic plants in soil. J Agron Crop Sci 191:162–171
Acknowledgement
K. Lavanya is thankful to Council of Scientific and Industrial Research (CSIR), New Delhi for a research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Batish, D.R., Lavanya, K., Singh, H.P. et al. Phenolic allelochemicals released by Chenopodium murale affect the growth, nodulation and macromolecule content in chickpea and pea. Plant Growth Regul 51, 119–128 (2007). https://doi.org/10.1007/s10725-006-9153-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-006-9153-z