Abstract
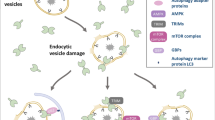
Sialylation is an important terminal modification of glycoconjugates that mediate diverse functions in physiology and disease. In this review we focus on how altered cell surface sialylation status is sensed by cytosolic galectins when the integrity of intracellular vesicles or organelles is compromised to expose luminal glycans to the cytosolic milieu, and how this impacts galectin-mediated cellular responses. In addition, we discuss the roles of mammalian sialidases on the cell surface, in the organelle lumen and cytosol, and raise the possibility that intracellular glycan processing may be critical in controlling various galectin-mediated responses when cells encounter stress.

Similar content being viewed by others
Data availability
Not applicable.
References
Liu, F.T., Rabinovich, G.A.: Galectins as modulators of tumour progression. Nat. Rev. Cancer. 5(1), 29–41 (2005). https://doi.org/10.1038/nrc1527
Hirabayashi, J., Hashidate, T., Arata, Y., Nishi, N., Nakamura, T., Hirashima, M., Urashima, T., Oka, T., Futai, M., Muller, W.E., Yagi, F., Kasai, K.: Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta. 1572(2–3), 232–254 (2002). https://doi.org/10.1016/s0304-4165(02)00311-2
Hughes, R.C.: Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim. Biophys. Acta. 1473(1), 172–185 (1999). https://doi.org/10.1016/s0304-4165(99)00177-4
Liu, F.T., Stowell, S.R.: The role of galectins in immunity and infection. Nat. Rev. Immunol. 1–16 (2023). https://doi.org/10.1038/s41577-022-00829-7
Nabi, I.R., Shankar, J., Dennis, J.W.: The galectin lattice at a glance. J. Cell. Sci. 128(13), 2213–2219 (2015). https://doi.org/10.1242/jcs.151159
Hong, M.H., Weng, I.C., Li, F.Y., Lin, W.H., Liu, F.T.: Intracellular galectins sense cytosolically exposed glycans as danger and mediate cellular responses. J. Biomed. Sci. 28(1), 16 (2021). https://doi.org/10.1186/s12929-021-00713-x
Liu, F.T., Patterson, R.J., Wang, J.L.: Intracellular functions of galectins. Biochim. Biophys. Acta. 1572(2–3), 263–273 (2002). https://doi.org/10.1016/s0304-4165(02)00313-6
Kelm, S., Schauer, R.: Sialic acids in molecular and cellular interactions. Int. Rev. Cytol. 175, 137–240 (1997). https://doi.org/10.1016/s0074-7696(08)62127-0
Lehmann, F., Tiralongo, E., Tiralongo, J.: Sialic acid-specific lectins: Occurrence, specificity and function. Cell. Mol. Life Sci. 63(12), 1331–1354 (2006). https://doi.org/10.1007/s00018-005-5589-y
Schauer, R.: Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 19(5), 507–514 (2009). https://doi.org/10.1016/j.sbi.2009.06.003
Varki, N.M., Varki, A.: Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab. Invest. 87(9), 851–857 (2007). https://doi.org/10.1038/labinvest.3700656
Lewis, A.L., Chen, X., Schnaar, R.L., Varki, A.: Sialic Acids and Other Nonulosonic Acids. In: Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., Schnaar, R.L., Seeberger, P.H. (eds.) Essentials of Glycobiology. pp. 185–204. Cold Spring Harbor Laboratory Press Copyright © 2022 The Consortium of Glycobiology Editors, La Jolla, California; published by Cold Spring Harbor Laboratory Press; doi: (2022). https://doi.org/10.1101/glycobiology.4e.15. All rights reserved., Cold Spring Harbor (NY)
Cavalcante, T., Medeiros, M.M., Mule, S.N., Palmisano, G., Stolf, B.S.: The role of sialic acids in the establishment of infections by pathogens, with Special Focus on Leishmania. Front. Cell. Infect. Microbiol. 11 (2021). https://doi.org/10.3389/fcimb.2021.671913
Stowell, S.R., Arthur, C.M., Mehta, P., Slanina, K.A., Blixt, O., Leffler, H., Smith, D.F., Cummings, R.D.: Galectin-1, -2, and – 3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 283(15), 10109–10123 (2008). https://doi.org/10.1074/jbc.M709545200
Zhuo, Y., Bellis, S.L.: Emerging role of alpha2,6-sialic acid as a negative regulator of galectin binding and function. J. Biol. Chem. 286(8), 5935–5941 (2011). https://doi.org/10.1074/jbc.R110.191429
Cagnoni, A.J., Troncoso, M.F., Rabinovich, G.A., Mariño, K.V., Elola, M.T.: Full-length galectin-8 and separate carbohydrate recognition domains: The whole is greater than the sum of its parts? Biochem. Soc. Trans. 48(3), 1255–1268 (2020). https://doi.org/10.1042/bst20200311
Stowell, S.R., Arthur, C.M., Slanina, K.A., Horton, J.R., Smith, D.F., Cummings, R.D.: Dimeric Galectin-8 induces phosphatidylserine exposure in Leukocytes through Polylactosamine Recognition by the C-terminal domain *. J. Biol. Chem. 283(29), 20547–20559 (2008). https://doi.org/10.1074/jbc.M802495200
Beatty, W.L., Rhoades, E.R., Hsu, D.K., Liu, F.T., Russell, D.G.: Association of a macrophage galactoside-binding protein with Mycobacterium-containing phagosomes. Cell. Microbiol. 4(3), 167–176 (2002). https://doi.org/10.1046/j.1462-5822.2002.00183.x
Dupont, N., Lacas-Gervais, S., Bertout, J., Paz, I., Freche, B., Van Nhieu, G.T., van der Goot, F.G., Sansonetti, P.J., Lafont, F.: Shigella phagocytic vacuolar membrane remnants participate in the cellular response to pathogen invasion and are regulated by autophagy. Cell. Host Microbe. 6(2), 137–149 (2009). https://doi.org/10.1016/j.chom.2009.07.005
Paz, I., Sachse, M., Dupont, N., Mounier, J., Cederfur, C., Enninga, J., Leffler, H., Poirier, F., Prevost, M.C., Lafont, F., Sansonetti, P.: Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell. Microbiol. 12(4), 530–544 (2010). https://doi.org/10.1111/j.1462-5822.2009.01415.x
Thurston, T.L., Wandel, M.P., von Muhlinen, N., Foeglein, A., Randow, F.: Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature. 482(7385), 414–418 (2012). https://doi.org/10.1038/nature10744
Cheng, Y.L., Wu, Y.W., Kuo, C.F., Lu, S.L., Liu, F.T., Anderson, R., Lin, C.F., Liu, Y.L., Wang, W.Y., Chen, Y.D., Zheng, P.X., Wu, J.J., Lin, Y.S.: Galectin-3 inhibits Galectin-8/Parkin-Mediated ubiquitination of Group A Streptococcus. mBio. 8(4) (2017). https://doi.org/10.1128/mBio.00899-17
Feeley, E.M., Pilla-Moffett, D.M., Zwack, E.E., Piro, A.S., Finethy, R., Kolb, J.P., Martinez, J., Brodsky, I.E., Coers, J.: Galectin-3 directs antimicrobial guanylate binding proteins to vacuoles furnished with bacterial secretion systems. Proc. Natl. Acad. Sci. U S A. 114(9), E1698–e1706 (2017). https://doi.org/10.1073/pnas.1615771114
Mansilla Pareja, M.E., Bongiovanni, A., Lafont, F., Colombo, M.I.: Alterations of the Coxiella burnetii replicative vacuole membrane Integrity and Interplay with the Autophagy Pathway. Front. Cell. Infect. Microbiol. 7, 112 (2017). https://doi.org/10.3389/fcimb.2017.00112
Weng, I.C., Chen, H.L., Lo, T.H., Lin, W.H., Chen, H.Y., Hsu, D.K., Liu, F.T.: Cytosolic galectin-3 and – 8 regulate antibacterial autophagy through differential recognition of host glycans on damaged phagosomes. Glycobiology. 28(6), 392–405 (2018). https://doi.org/10.1093/glycob/cwy017
Li, F.Y., Weng, I.C., Lin, C.H., Kao, M.C., Wu, M.S., Chen, H.Y., Liu, F.T.: Helicobacter pylori induces intracellular galectin-8 aggregation around damaged lysosomes within gastric epithelial cells in a host O-glycan-dependent manner. Glycobiology. 29(2), 151–162 (2019). https://doi.org/10.1093/glycob/cwy095
Maier, O., Marvin, S.A., Wodrich, H., Campbell, E.M., Wiethoff, C.M.: Spatiotemporal dynamics of adenovirus membrane rupture and endosomal escape. J. Virol. 86(19), 10821–10828 (2012). https://doi.org/10.1128/jvi.01428-12
Montespan, C., Marvin, S.A., Austin, S., Burrage, A.M., Roger, B., Rayne, F., Faure, M., Campell, E.M., Schneider, C., Reimer, R., Grünewald, K., Wiethoff, C.M., Wodrich, H.: Multi-layered control of Galectin-8 mediated autophagy during adenovirus cell entry through a conserved PPxY motif in the viral capsid. PLoS Pathog. 13(2) (2017). https://doi.org/10.1371/journal.ppat.1006217 e1006217
Staring, J., von Castelmur, E., Blomen, V.A., van den Hengel, L.G., Brockmann, M., Baggen, J., Thibaut, H.J., Nieuwenhuis, J., Janssen, H., van Kuppeveld, F.J., Perrakis, A., Carette, J.E., Brummelkamp, T.R.: PLA2G16 represents a switch between entry and clearance of Picornaviridae. Nature. 541(7637), 412–416 (2017). https://doi.org/10.1038/nature21032
Falcon, B., Noad, J., McMahon, H., Randow, F., Goedert, M.: Galectin-8-mediated selective autophagy protects against seeded tau aggregation. J. Biol. Chem. 293(7), 2438–2451 (2018). https://doi.org/10.1074/jbc.M117.809293
Freeman, D., Cedillos, R., Choyke, S., Lukic, Z., McGuire, K., Marvin, S., Burrage, A.M., Sudholt, S., Rana, A., O’Connor, C., Wiethoff, C.M., Campbell, E.M.: Alpha-synuclein induces lysosomal rupture and cathepsin dependent reactive oxygen species following endocytosis. PLoS One. 8(4) (2013). https://doi.org/10.1371/journal.pone.0062143 e62143
Siew, J.J., Chen, H.-M., Chen, H.-Y., Chen, H.-L., Chen, C.-M., Soong, B.-W., Wu, Y.-R., Chang, C.-P., Chan, Y.-C., Lin, C.-H., Liu, F.-T., Chern, Y.: Galectin-3 is required for the microglia-mediated brain inflammation in a model of Huntington’s disease. Nat. Commun. 10(1), 3473 (2019). https://doi.org/10.1038/s41467-019-11441-0
Maejima, I., Takahashi, A., Omori, H., Kimura, T., Takabatake, Y., Saitoh, T., Yamamoto, A., Hamasaki, M., Noda, T., Isaka, Y., Yoshimori, T.: Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. Embo j. 32(17), 2336–2347 (2013). https://doi.org/10.1038/emboj.2013.171
Unno, R., Kawabata, T., Taguchi, K., Sugino, T., Hamamoto, S., Ando, R., Okada, A., Kohri, K., Yoshimori, T., Yasui, T.: Deregulated MTOR (mechanistic target of rapamycin kinase) is responsible for autophagy defects exacerbating kidney stone development. Autophagy. 16(4), 709–723 (2020). https://doi.org/10.1080/15548627.2019.1635382
Wittrup, A., Ai, A., Liu, X., Hamar, P., Trifonova, R., Charisse, K., Manoharan, M., Kirchhausen, T., Lieberman, J.: Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 33(8), 870–876 (2015). https://doi.org/10.1038/nbt.3298
Chen, X., Khambu, B., Zhang, H., Gao, W., Li, M., Chen, X., Yoshimori, T., Yin, X.M.: Autophagy induced by calcium phosphate precipitates targets damaged endosomes. J. Biol. Chem. 289(16), 11162–11174 (2014). https://doi.org/10.1074/jbc.M113.531855
Kilchrist, K.V., Dimobi, S.C., Jackson, M.A., Evans, B.C., Werfel, T.A., Dailing, E.A., Bedingfield, S.K., Kelly, I.B., Duvall, C.L.: Gal8 visualization of endosome disruption predicts carrier-mediated Biologic Drug Intracellular Bioavailability. ACS Nano. 13(2), 1136–1152 (2019). https://doi.org/10.1021/acsnano.8b05482
Jia, J., Abudu, Y.P., Claude-Taupin, A., Gu, Y., Kumar, S., Choi, S.W., Peters, R., Mudd, M.H., Allers, L., Salemi, M., Phinney, B., Johansen, T., Deretic, V.: Galectins Control mTOR in response to endomembrane damage. Mol. Cell. 70(1), 120–135e128 (2018). https://doi.org/10.1016/j.molcel.2018.03.009
Chen, X., Khambu, B., Zhang, H., Gao, W., Li, M., Chen, X., Yoshimori, T., Yin, X.-M.: Autophagy Induced by Calcium phosphate precipitates targets damaged Endosomes *. J. Biol. Chem. 289(16), 11162–11174 (2014). https://doi.org/10.1074/jbc.M113.531855
Chauhan, S., Kumar, S., Jain, A., Ponpuak, M., Mudd, M.H., Kimura, T., Choi, S.W., Peters, R., Mandell, M., Bruun, J.A., Johansen, T., Deretic, V.: TRIMs and galectins globally cooperate and TRIM16 and Galectin-3 co-direct autophagy in endomembrane damage homeostasis. Dev. Cell. 39(1), 13–27 (2016). https://doi.org/10.1016/j.devcel.2016.08.003
Lewis, A.L., Lewis, W.G.: Host sialoglycans and bacterial sialidases: A mucosal perspective. Cell. Microbiol. 14(8), 1174–1182 (2012). https://doi.org/10.1111/j.1462-5822.2012.01807.x
Huang, Y.L., Chassard, C., Hausmann, M., von Itzstein, M., Hennet, T.: Sialic acid catabolism drives intestinal inflammation and microbial dysbiosis in mice. Nat. Commun. 6, 8141 (2015). https://doi.org/10.1038/ncomms9141
Morosi, L.G., Cutine, A.M., Cagnoni, A.J., Manselle-Cocco, M.N., Croci, D.O., Merlo, J.P., Morales, R.M., May, M., Pérez-Sáez, J.M., Girotti, M.R., Méndez-Huergo, S.P., Pucci, B., Gil, A.H., Huernos, S.P., Docena, G.H., Sambuelli, A.M., Toscano, M.A., Rabinovich, G.A., Mariño, K.V.: Control of intestinal inflammation by glycosylation-dependent lectin-driven immunoregulatory circuits. Sci. Adv. 7(25) (2021). https://doi.org/10.1126/sciadv.abf8630
Yang, W.H., Westman, J.S., Heithoff, D.M., Sperandio, M., Cho, J.W., Mahan, M.J., Marth, J.D.: Neu3 neuraminidase induction triggers intestinal inflammation and colitis in a model of recurrent human food-poisoning. Proc. Natl. Acad. Sci. U S A. 118(29) (2021). https://doi.org/10.1073/pnas.2100937118
McAuley, J.L., Gilbertson, B.P., Trifkovic, S., Brown, L.E., McKimm-Breschkin, J.L.: Influenza Virus Neuraminidase structure and functions. Front. Microbiol. 10 (2019). https://doi.org/10.3389/fmicb.2019.00039
Suzuki, T., Takahashi, T., Guo, C.T., Hidari, K.I., Miyamoto, D., Goto, H., Kawaoka, Y., Suzuki, Y.: Sialidase activity of influenza a virus in an endocytic pathway enhances viral replication. J. Virol. 79(18), 11705–11715 (2005). https://doi.org/10.1128/jvi.79.18.11705-11715.2005
Villanueva, M.S., Beckers, C.J., Pamer, E.G.: Infection with Listeria monocytogenes impairs sialic acid addition to host cell glycoproteins. J. Exp. Med. 180(6), 2137–2145 (1994). https://doi.org/10.1084/jem.180.6.2137
Flieger, A., Frischknecht, F., Häcker, G., Hornef, M.W., Pradel, G.: Pathways of host cell exit by intracellular pathogens. Microb. Cell. 5(12), 525–544 (2018). https://doi.org/10.15698/mic2018.12.659
Rubin-de-Celis, S.S., Uemura, H., Yoshida, N., Schenkman, S.: Expression of trypomastigote trans-sialidase in metacyclic forms of Trypanosoma cruzi increases parasite escape from its parasitophorous vacuole. Cell. Microbiol. 8(12), 1888–1898 (2006). https://doi.org/10.1111/j.1462-5822.2006.00755.x
Freire-de-Lima, L., da Fonseca, L.M., da Silva, V.A., da Costa, K.M., Morrot, A., Freire-de-Lima, C.G., Previato, J.O., Mendonça-Previato, L.: Modulation of cell sialoglycophenotype: A stylish mechanism adopted by Trypanosoma cruzi to ensure its persistence in the infected host. Front. Microbiol. 7, 698 (2016). https://doi.org/10.3389/fmicb.2016.00698
Puigdellívol Cañadell, M., Allendorf, D., Brown, G.: Sialylation and Galectin-3 in microglia-mediated neuroinflammation and neurodegeneration. Front. Cell. Neurosci. 14 (2020). https://doi.org/10.3389/fncel.2020.00162
Desplats, P.A., Denny, C.A., Kass, K.E., Gilmartin, T., Head, S.R., Sutcliffe, J.G., Seyfried, T.N., Thomas, E.A.: Glycolipid and ganglioside metabolism imbalances in Huntington’s disease. Neurobiol. Dis. 27(3), 265–277 (2007). https://doi.org/10.1016/j.nbd.2007.05.003
Hong, M.H., Lin, W.H., Weng, I.C., Hung, Y.H., Chen, H.L., Chen, H.Y., Chen, P., Lin, C.H., Yang, W.Y., Liu, F.T.: Intracellular galectins control cellular responses commensurate with cell surface carbohydrate composition. Glycobiology. 30(1), 49–57 (2019). https://doi.org/10.1093/glycob/cwz075
Ideo, H., Matsuzaka, T., Nonaka, T., Seko, A., Yamashita, K.: Galectin-8-N-domain recognition mechanism for sialylated and sulfated glycans. J. Biol. Chem. 286(13), 11346–11355 (2011). https://doi.org/10.1074/jbc.M110.195925
Glanz, V.Y., Myasoedova, V.A., Grechko, A.V., Orekhov, A.N.: Sialidase activity in human pathologies. Eur. J. Pharmacol. 842, 345–350 (2019). https://doi.org/10.1016/j.ejphar.2018.11.014
Pshezhetsky, A.V., Hinek, A.: Where catabolism meets signalling: Neuraminidase 1 as a modulator of cell receptors. Glycoconj. J. 28(7), 441–452 (2011). https://doi.org/10.1007/s10719-011-9350-5
Pshezhetsky, A.V., Ashmarina, L.I.: Desialylation of surface receptors as a new dimension in cell signaling. Biochem. (Mosc). 78(7), 736–745 (2013). https://doi.org/10.1134/s0006297913070067
Mozzi, A., Forcella, M., Riva, A., Difrancesco, C., Molinari, F., Martin, V., Papini, N., Bernasconi, B., Nonnis, S., Tedeschi, G., Mazzucchelli, L., Monti, E., Fusi, P., Frattini, M.: NEU3 activity enhances EGFR activation without affecting EGFR expression and acts on its sialylation levels. Glycobiology. 25(8), 855–868 (2015). https://doi.org/10.1093/glycob/cwv026
Zhang, X., Dou, P., Akhtar, M.L., Liu, F., Hu, X., Yang, L., Yang, D., Zhang, X., Li, Y., Qiao, S., Li, K., Tang, R., Zhan, C., Ma, Y., Cheng, Q., Bai, Y., Han, F., Nie, H., Li, Y.: NEU4 inhibits motility of HCC cells by cleaving sialic acids on CD44. Oncogene. 40(35), 5427–5440 (2021). https://doi.org/10.1038/s41388-021-01955-7
Shiozaki, K., Yamaguchi, K., Takahashi, K., Moriya, S., Miyagi, T.: Regulation of sialyl Lewis antigen expression in colon cancer cells by sialidase NEU4. J. Biol. Chem. 286(24), 21052–21061 (2011). https://doi.org/10.1074/jbc.M111.231191
Takahashi, K., Mitoma, J., Hosono, M., Shiozaki, K., Sato, C., Yamaguchi, K., Kitajima, K., Higashi, H., Nitta, K., Shima, H., Miyagi, T.: Sialidase NEU4 hydrolyzes polysialic acids of neural cell adhesion molecules and negatively regulates neurite formation by hippocampal neurons. J. Biol. Chem. 287(18), 14816–14826 (2012). https://doi.org/10.1074/jbc.M111.324186
Yogalingam, G., Bonten, E.J., van de Vlekkert, D., Hu, H., Moshiach, S., Connell, S.A., d’Azzo, A.: Neuraminidase 1 is a negative regulator of lysosomal exocytosis. Dev. Cell. 15(1), 74–86 (2008). https://doi.org/10.1016/j.devcel.2008.05.005
Seyrantepe, V., Poupetova, H., Froissart, R., Zabot, M.T., Maire, I., Pshezhetsky, A.V.: Molecular pathology of NEU1 gene in sialidosis. Hum. Mutat. 22(5), 343–352 (2003). https://doi.org/10.1002/humu.10268
Lukong, K.E., Seyrantepe, V., Landry, K., Trudel, S., Ahmad, A., Gahl, W.A., Lefrancois, S., Morales, C.R., Pshezhetsky, A.V.: Intracellular distribution of lysosomal sialidase is controlled by the internalization signal in its cytoplasmic tail. J. Biol. Chem. 276(49), 46172–46181 (2001). https://doi.org/10.1074/jbc.M104547200
Annunziata, I., Patterson, A., Helton, D., Hu, H., Moshiach, S., Gomero, E., Nixon, R., d’Azzo, A.: Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-β secretion via deregulated lysosomal exocytosis. Nat. Commun. 4, 2734 (2013). https://doi.org/10.1038/ncomms3734
Abdulkhalek, S., Szewczuk, M.R.: Neu1 sialidase and matrix metalloproteinase-9 cross-talk regulates nucleic acid-induced endosomal TOLL-like receptor-7 and – 9 activation, cellular signaling and pro-inflammatory responses. Cell. Signal. 25(11), 2093–2105 (2013). https://doi.org/10.1016/j.cellsig.2013.06.010
Miyagi, T., Yamamoto, K.: Review sialidase NEU3 and its pathological significance. Glycoconj. J. (2022). https://doi.org/10.1007/s10719-022-10067-7
Ballabio, A., Gieselmann, V.: Lysosomal disorders: From storage to cellular damage. Biochim. Biophys. Acta. 1793(4), 684–696 (2009). https://doi.org/10.1016/j.bbamcr.2008.12.001
New-Aaron, M., Thomes, P.G., Ganesan, M., Dagur, R.S., Donohue, T.M. Jr., Kusum, K.K., Poluektova, L.Y., Osna, N.A.: Alcohol-Induced lysosomal damage and suppression of Lysosome Biogenesis Contribute to Hepatotoxicity in HIV-Exposed liver cells. Biomolecules. 11(10) (2021). https://doi.org/10.3390/biom11101497
Oku, Y., Murakami, K., Irie, K., Hoseki, J., Sakai, Y.: Synthesized Aβ42 caused intracellular oxidative damage, leading to cell death, via Lysosome rupture. Cell. Struct. Funct. 42(1), 71–79 (2017). https://doi.org/10.1247/csf.17006
Cai, B.H., Wu, P.H., Chou, C.K., Huang, H.C., Chao, C.C., Chung, H.Y., Lee, H.Y., Chen, J.Y., Kannagi, R.: Synergistic activation of the NEU4 promoter by p73 and AP2 in colon cancer cells. Sci. Rep. 9(1), 950 (2019). https://doi.org/10.1038/s41598-018-37521-7
Reily, C., Stewart, T.J., Renfrow, M.B., Novak, J.: Glycosylation in health and disease. Nat. Rev. Nephrol. 15(6), 346–366 (2019). https://doi.org/10.1038/s41581-019-0129-4
Funding
This work was supported by Academia Sinica and the Ministry of Science and Technology in Taiwan (MOST 109-2320-B-001-024-MY3).
Author information
Authors and Affiliations
Contributions
ICW and HLC wrote the manuscript, FTL edited the manuscript, WHL prepared the figure. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weng, IC., Chen, HL., Lin, WH. et al. Sialylation of cell surface glycoconjugates modulates cytosolic galectin-mediated responses upon organelle damage. Glycoconj J 40, 295–303 (2023). https://doi.org/10.1007/s10719-023-10112-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-023-10112-z