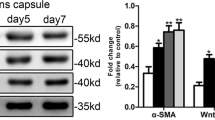
Abstract
Basement membrane (BM) proteins accumulate chemical modifications with age. One such modification is glycation, which results in the formation of advanced glycation endproducts (AGEs). In a previous study, we reported that AGEs in the human lens capsule (BM) promote the TGFβ2-mediated epithelial-to-mesenchymal transition (EMT) of lens epithelial cells, which we proposed as a mechanism for posterior capsule opacification (PCO) or secondary cataract formation. In this study, we investigated the role of a receptor for AGEs (RAGE) in the TGFβ2-mediated EMT in a human lens epithelial cell line (FHL124). RAGE was present in FHL124 cells, and its levels were unaltered in cells cultured on either native or AGE-modified BM or upon treatment with TGFβ2. RAGE overexpression significantly enhanced the TGFβ2-mediated EMT responses in cells cultured on AGE-modified BM compared with the unmodified matrix. In contrast, treatment of cells with a RAGE antibody or EN-RAGE (an endogenous ligand for RAGE) resulted in a significant reduction in the TGFβ2-mediated EMT response. This was accompanied by a reduction in TGFβ2-mediated Smad signaling and ROS generation. These results imply that the interaction of matrix AGEs with RAGE plays a role in the TGFβ2-mediated EMT of lens epithelial cells and suggest that the blockade of RAGE could be a strategy to prevent PCO and other age-associated fibrosis.







Similar content being viewed by others
Abbreviations
- AGEs:
-
advanced glycation endproducts
- BM:
-
basement membrane
- BME:
-
basement membrane extract
- EMT:
-
epithelial to mesenchymal transition
- FBS:
-
fetal bovine serum
- MEM:
-
minimum essential medium
- PCO:
-
posterior capsule opacification
- RAGE:
-
receptor for advanced glycation endproducts
- ROS:
-
reactive oxygen species
- αSMA:
-
alpha smooth muscle actin
- CTGF:
-
connective tissue growth factor
- TGFβ2:
-
transforming growth factor beta2
- ZO-1:
-
zona occuldin-1
References
Sell D.R., Monnier V.M.: Aging of long-lived proteins: extra-cellular matrix (collagens, elastins, proteoglycans) and lens crystallins. In: Masoro E.J. (ed.) Handbook of physiology. Section 11: aging, pp. 235–305. Oxford University Press, New York (1995)
Nagaraj R.H., Linetsky M., Stitt A.W.: The pathogenic role of Maillard reaction in the aging eye. Amino Acids. 42(4), 1205–1220 (2012)
Ahmed N., Thornalley P.J.: Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab. 9(3), 233–245 (2007)
Raghavan C.T., Smuda M., Smith A.J., Howell S., Smith D.G., Singh A., Gupta P., Glomb M.A., Wormstone I.M., Nagaraj R.H.: AGEs in human lens capsule promote the TGFbeta2-mediated EMT of lens epithelial cells: implications for age-associated fibrosis. Aging Cell. 15(3), 465–476 (2016)
Pascolini D., Mariotti S.P.: Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 96(5), 614–618 (2012)
Vision 2020: the cataract challenge. Community Eye Health 13(34), 17–19 (2000).
Awasthi N., Guo S., Wagner B.J.: Posterior capsular opacification: a problem reduced but not yet eradicated. Arch. Ophthalmol. 127(4), 555–562 (2009)
Wormstone I.M., Wang L., Liu C.S.: Posterior capsule opacification. Exp. Eye Res. 88(2), 257–269 (2009)
Marcantonio J.M., Syam P.P., Liu C.S., Duncan G.: Epithelial transdifferentiation and cataract in the human lens. Exp. Eye Res. 77(3), 339–346 (2003)
Billotte C., Berdeaux G.: Adverse clinical consequences of neodymium:YAG laser treatment of posterior capsule opacification. J. Cataract Refract. Surg. 30(10), 2064–2071 (2004)
Trinavarat A., Atchaneeyasakul L., Udompunturak S.: Neodymium:YAG laser damage threshold of foldable intraocular lenses. J. Cataract Refract. Surg. 27(5), 775–780 (2001)
Jampel H.D., Roche N., Stark W.J., Roberts A.B.: Transforming growth factor-beta in human aqueous humor. Curr. Eye Res. 9(10), 963–969 (1990)
Ohta K., Yamagami S., Taylor A.W., Streilein J.W.: IL-6 antagonizes TGF-beta and abolishes immune privilege in eyes with endotoxin-induced uveitis. Invest. Ophthalmol. Vis. Sci. 41(9), 2591–2599 (2000)
de Iongh R.U., Wederell E., Lovicu F.J., McAvoy J.W.: Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 179(1–2), 43–55 (2005)
Li J., Tang X., Chen X.: Comparative effects of TGF-beta2/Smad2 and TGF-beta2/Smad3 signaling pathways on proliferation, migration, and extracellular matrix production in a human lens cell line. Exp. Eye Res. 92(3), 173–179 (2011)
Saika S., Kono-Saika S., Ohnishi Y., Sato M., Muragaki Y., Ooshima A., Flanders K.C., Yoo J., Anzano M., Liu C.Y., Kao W.W., Roberts A.B.: Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am. J. Pathol. 164(2), 651–663 (2004)
Wang Y., Li W., Zang X., Chen N., Liu T., Tsonis P.A., Huang Y.: MicroRNA-204-5p regulates epithelial-to-mesenchymal transition during human posterior capsule opacification by targeting SMAD4. Invest. Ophthalmol. Vis. Sci. 54(1), 323–332 (2013)
Wormstone I.M., Tamiya S., Eldred J.A., Lazaridis K., Chantry A., Reddan J.R., Anderson I., Duncan G.: Characterisation of TGF-beta2 signalling and function in a human lens cell line. Exp. Eye Res. 78(3), 705–714 (2004)
Chen X., Ye S., Xiao W., Wang W., Luo L., Liu Y.: ERK1/2 pathway mediates epithelial-mesenchymal transition by cross-interacting with TGFbeta/Smad and jagged/notch signaling pathways in lens epithelial cells. Int. J. Mol. Med. 33(6), 1664–1670 (2014)
Lovicu F.J., McAvoy J.W., de Iongh R.U.: Understanding the role of growth factors in embryonic development: insights from the lens. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 366(1568), 1204–1218 (2011)
Burns W.C., Twigg S.M., Forbes J.M., Pete J., Tikellis C., Thallas-Bonke V., Thomas M.C., Cooper M.E., Kantharidis P.: Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: implications for diabetic renal disease. J. Am. Soc. Nephrol. 17(9), 2484–2494 (2006)
Chen L., Wang T., Wang X., Sun B.B., Li J.Q., Liu D.S., Zhang S.F., Liu L., Xu D., Chen Y.J., Wen F.Q.: Blockade of advanced glycation end product formation attenuates bleomycin-induced pulmonary fibrosis in rats. Respir. Res. 10, 55 (2009)
Neeper M., Schmidt A.M., Brett J., Yan S.D., Wang F., Pan Y.C., Elliston K., Stern D., Shaw A.: Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 267(21), 14998–15004 (1992)
Hori O., Brett J., Slattery T., Cao R., Zhang J., Chen J.X., Nagashima M., Lundh E.R., Vijay S., Nitecki D., et al.: The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J. Biol. Chem. 270(43), 25752–25761 (1995)
Leclerc E., Fritz G., Vetter S.W., Heizmann C.W.: Binding of S100 proteins to RAGE: an update. Biochim. Biophys. Acta. 1793(6), 993–1007 (2009)
Xie J., Burz D.S., He W., Bronstein I.B., Lednev I., Shekhtman A.: Hexameric calgranulin C (S100 A12) binds to the receptor for advanced glycated end products (RAGE) using symmetric hydrophobic target-binding patches. J. Biol. Chem. 282(6), 4218–4231 (2007)
Dattilo B.M., Fritz G., Leclerc E., Kooi C.W., Heizmann C.W., Chazin W.J.: The extracellular region of the receptor for advanced glycation end products is composed of two independent structural units. Biochemistry. 46(23), 6957–6970 (2007)
Ostendorp T., Leclerc E., Galichet A., Koch M., Demling N., Weigle B., Heizmann C.W., Kroneck P.M., Fritz G.: Structural and functional insights into RAGE activation by multimeric S100B. EMBO J. 26(16), 3868–3878 (2007)
Leclerc E., Fritz G., Weibel M., Heizmann C.W., Galichet A.: S100B and S100 A6 differentially modulate cell survival by interacting with distinct RAGE (receptor for advanced glycation end products) immunoglobulin domains. J. Biol. Chem. 282(43), 31317–31331 (2007)
Sturchler E., Galichet A., Weibel M., Leclerc E., Heizmann C.W.: Site-specific blockade of RAGE-Vd prevents amyloid-beta oligomer neurotoxicity. J. Neurosci. 28(20), 5149–5158 (2008)
Kislinger T., Fu C., Huber B., Qu W., Taguchi A., Du Yan S., Hofmann M., Yan S.F., Pischetsrieder M., Stern D., Schmidt A.M.: N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J. Biol. Chem. 274(44), 31740–31749 (1999)
Xie J., Reverdatto S., Frolov A., Hoffmann R., Burz D.S., Shekhtman A.: Structural basis for pattern recognition by the receptor for advanced glycation end products (RAGE). J. Biol. Chem. 283(40), 27255–27269 (2008)
Lander H.M., Tauras J.M., Ogiste J.S., Hori O., Moss R.A., Schmidt A.M.: Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J. Biol. Chem. 272(28), 17810–17814 (1997)
Ramasamy R., Vannucci S.J., Yan S.S., Herold K., Yan S.F., Schmidt A.M.: Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 15(7), 16R–28R (2005)
Lue L.F., Walker D.G., Brachova L., Beach T.G., Rogers J., Schmidt A.M., Stern D.M., Yan S.D.: Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Exp. Neurol. 171(1), 29–45 (2001)
Sasahira T., Kirita T., Bhawal U.K., Yamamoto K., Ohmori H., Fujii K., Kuniyasu H.: Receptor for advanced glycation end products (RAGE) is important in the prediction of recurrence in human oral squamous cell carcinoma. Histopathology. 51(2), 166–172 (2007)
Wang Y., Vom Hagen F., Pfister F., Bierhaus A., Feng Y., Gans R., Hammes H.P.: Receptor for advanced glycation end product expression in experimental diabetic retinopathy. Ann. N. Y. Acad. Sci. 1126, 42–45 (2008)
Howes K.A., Liu Y., Dunaief J.L., Milam A., Frederick J.M., Marks A., Baehr W.: Receptor for advanced glycation end products and age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 45(10), 3713–3720 (2004)
Wautier M.P., Chappey O., Corda S., Stern D.M., Schmidt A.M., Wautier J.L.: Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 280(5), E685–E694 (2001)
Mizumoto S., Takahashi J., Sugahara K.: Receptor for advanced glycation end products (RAGE) functions as receptor for specific sulfated glycosaminoglycans, and anti-RAGE antibody or sulfated glycosaminoglycans delivered in vivo inhibit pulmonary metastasis of tumor cells. J. Biol. Chem. 287(23), 18985–18994 (2012)
Deane R., Singh I., Sagare A.P., Bell R.D., Ross N.T., LaRue B., Love R., Perry S., Paquette N., Deane R.J., Thiyagarajan M., Zarcone T., Fritz G., Friedman A.E., Miller B.L., Zlokovic B.V.: A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Invest. 122(4), 1377–1392 (2012)
Ganatra D.A., Rajkumar S., Patel A.R., Gajjar D.U., Johar K., Arora A.I., Kayastha F.B., Vasavada A.R.: Association of histone acetylation at the ACTA2 promoter region with epithelial mesenchymal transition of lens epithelial cells. Eye (Lond). 29(6), 828–838 (2015)
Fehrenbach H., Weiskirchen R., Kasper M., Gressner A.M.: Up-regulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during transdifferentiation to myofibroblasts. Hepatology. 34(5), 943–952 (2001)
Serban A.I., Stanca L., Geicu O.I., Munteanu M.C., Costache M., Dinischiotu A.: Extracellular matrix is modulated in advanced glycation end products milieu via a RAGE receptor dependent pathway boosted by transforming growth factor-beta1 RAGE. J Diabetes. 7(1), 114–124 (2015)
Huang K.P., Chen C., Hao J., Huang J.Y., Liu P.Q., Huang H.Q.: AGEs-RAGE system down-regulates Sirt1 through the ubiquitin-proteasome pathway to promote FN and TGF-beta1 expression in male rat glomerular mesangial cells. Endocrinology. 156(1), 268–279 (2015)
Fang M., Wang J., Li S., Guo Y.: Advanced glycation end-products accelerate the cardiac aging process through the receptor for advanced glycation end-products/transforming growth factor-beta-Smad signaling pathway in cardiac fibroblasts. Geriatr. Gerontol. Int. 16(4), 522–527 (2016)
Yamagishi S., Inagaki Y., Okamoto T., Amano S., Koga K., Takeuchi M.: Advanced glycation end products inhibit de novo protein synthesis and induce TGF-beta overexpression in proximal tubular cells. Kidney Int. 63(2), 464–473 (2003)
Li P., Chen G.R., Wang F., Xu P., Liu L.Y., Yin Y.L., Wang S.X.: Inhibition of NA(+)/H(+) exchanger 1 attenuates renal dysfunction induced by advanced glycation end products in rats. J Diabetes Res. 2016, 1802036 (2016)
Cheng M., Liu H., Zhang D., Liu Y., Wang C., Liu F., Chen J.: HMGB1 enhances the AGE-induced expression of CTGF and TGF-beta via RAGE-dependent signaling in renal tubular epithelial cells. Am. J. Nephrol. 41(3), 257–266 (2015)
Yu L., Zhao Y., Xu S., Ding F., Jin C., Fu G., Weng S.: Advanced glycation end product (AGE)-AGE receptor (RAGE) system upregulated Connexin43 expression in rat cardiomyocytes via PKC and Erk MAPK pathways. Int. J. Mol. Sci. 14(2), 2242–2257 (2013)
Li J.H., Huang X.R., Zhu H.J., Oldfield M., Cooper M., Truong L.D., Johnson R.J., Lan H.Y.: Advanced glycation end products activate Smad signaling via TGF-beta-dependent and independent mechanisms: implications for diabetic renal and vascular disease. FASEB J. 18(1), 176–178 (2004)
Yamashita H., ten Dijke P., Franzen P., Miyazono K., Heldin C.H.: Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-beta. J. Biol. Chem. 269(31), 20172–20178 (1994)
Brizzi M.F., Dentelli P., Rosso A., Calvi C., Gambino R., Cassader M., Salvidio G., Deferrari G., Camussi G., Pegoraro L., Pagano G., Cavallo-Perin P.: RAGE- and TGF-beta receptor-mediated signals converge on STAT5 and p21waf to control cell-cycle progression of mesangial cells: a possible role in the development and progression of diabetic nephropathy. FASEB J. 18(11), 1249–1251 (2004)
Yamagishi S., Matsui T.: Role of receptor for advanced glycation end products (RAGE) in liver disease. Eur. J. Med. Res. 20, 15 (2015)
Zhao J., Randive R., Stewart J.A.: Molecular mechanisms of AGE/RAGE-mediated fibrosis in the diabetic heart. World J Diabetes. 5(6), 860–867 (2014)
Russo I., Frangogiannis N.G.: Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. 90, 84–93 (2016)
Li J.H., Wang W., Huang X.R., Oldfield M., Schmidt A.M., Cooper M.E., Lan H.Y.: Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am. J. Pathol. 164(4), 1389–1397 (2004)
Qi W., Niu J., Qin Q., Qiao Z., Gu Y.: Glycated albumin triggers fibrosis and apoptosis via an NADPH oxidase/Nox4-MAPK pathway-dependent mechanism in renal proximal tubular cells. Mol. Cell. Endocrinol. 405, 74–83 (2015)
He W., Zhang J., Gan T.Y., Xu G.J., Tang B.P.: Advanced glycation end products induce endothelial-to-mesenchymal transition via downregulating Sirt 1 and upregulating TGF-beta in human endothelial cells. Biomed. Res. Int. 2015, 684242 (2015)
Ding H., Ji X., Chen R., Ma T., Tang Z., Fen Y., Cai H.: Antifibrotic properties of receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther. 35, 34–41 (2015)
Englert J.M., Kliment C.R., Ramsgaard L., Milutinovic P.S., Crum L., Tobolewski J.M., Oury T.D.: Paradoxical function for the receptor for advanced glycation end products in mouse models of pulmonary fibrosis. Int. J. Clin. Exp. Pathol. 4(3), 241–254 (2011)
Piperi C., Goumenos A., Adamopoulos C., Papavassiliou A.G.: AGE/RAGE signalling regulation by miRNAs: associations with diabetic complications and therapeutic potential. Int. J. Biochem. Cell Biol. 60, 197–201 (2015)
Lopez-Novoa J.M., Nieto M.A.: Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 1(6–7), 303–314 (2009)
Cheresh P., Kim S.J., Tulasiram S., Kamp D.W.: Oxidative stress and pulmonary fibrosis. Biochim. Biophys. Acta. 1832(7), 1028–1040 (2013)
Cichon M.A., Radisky D.C.: ROS-induced epithelial-mesenchymal transition in mammary epithelial cells is mediated by NF-kB-dependent activation of snail. Oncotarget. 5(9), 2827–2838 (2014)
Hong Y., Shen C., Yin Q., Sun M., Ma Y., Liu X.: Effects of RAGE-specific inhibitor FPS-ZM1 on amyloid-beta metabolism and AGEs-induced inflammation and oxidative stress in rat hippocampus. Neurochem. Res. 41(5), 1192–1199 (2016)
Dawes L.J., Elliott R.M., Reddan J.R., Wormstone Y.M., Wormstone I.M.: Oligonucleotide microarray analysis of human lens epithelial cells: TGFbeta regulated gene expression. Mol. Vis. 13, 1181–1197 (2007)
Spandidos A., Wang X., Wang H., Seed B.: PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 38(Database issue), D792–D799 (2010)
Acknowledgments
This work was supported by the National Institutes of Health Grants EY022061, EY023286 (to RHN). We thank Drs. Rooban Nahomi, Johanna Rankenberg and Stefan Rakete for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Raghavan, C.T., Nagaraj, R.H. AGE-RAGE interaction in the TGFβ2-mediated epithelial to mesenchymal transition of human lens epithelial cells. Glycoconj J 33, 631–643 (2016). https://doi.org/10.1007/s10719-016-9686-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-016-9686-y