Abstract
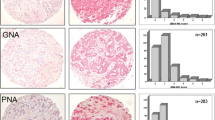
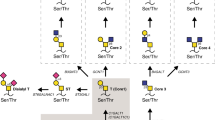
Glycan structure alterations during cancer regulate disease progression and represent clinical biomarkers. The study determined the degree to which changes in glycosyltransferase activities during cancer can be related to aberrant cell-surface tumor associated carbohydrate structures (TACA). To this end, changes in sialyltransferase (sialylT), fucosyltransferase (fucT) and galactosyltransferase (galT) activity were measured in normal and tumor tissue using a miniaturized enzyme activity assay and synthetic glycoconjugates bearing terminal LacNAc Type-I (Galβ1-3GlcNAc), LacNAc Type-II (Galβ1-4GlcNAc), and mucin core-1/Type-III (Galβ1-3GalNAc) structures. These data were related to TACA using tissue microarrays containing 115 breast and 26 colon cancer specimen. The results show that primary human breast and colon tumors, but not adjacent normal tissue, express elevated β1,3GalT and α2,3SialylT activity that can form α2,3SialylatedType-IIIglycans (Siaα2-3Galβ1-3GalNAc). Prostate tumors did not exhibit such elevated enzymatic activities. α1,3/4FucT activity was higher in breast, but not in colon tissue. The enzymology based prediction of enhanced α2,3sialylated Type-III structures in breast tumors was verified using histochemical analysis of tissue sections and tissue microarrays. Here, the binding of two markers that recognize Galβ1-3GalNAc (peanut lectin and mAb A78-G/A7) was elevated in breast tumor, but not in normal control, only upon sialidase treatment. These antigens were also upregulated in colon tumors though to a lesser extent. α2,3sialylatedType-III expression correlated inversely with patient HER2 expression and breast metastatic potential. Overall, enzymology measurements of glycoT activity predict truncated O-glycan structures in tumors. High expression of the α2,3sialylated T-antigen O-glycans occur in breast tumors. A transformation from linear core-1 glycan to other epitopes may accompany metastasis.





Similar content being viewed by others
Abbreviations
- GlycoT:
-
Glycosyltransferase
- Galβ1-3GalNAc:
-
Type-III or core-1 type acceptors
- Galβ1-4GlcNAc:
-
Type II or LacNAc type chain
- GalT:
-
Galactosyltransferase
- FT or FucT:
-
Fucosyltransferase
- SialylT:
-
Sialyltransferase
- ST3[Galβ1,3GalNAc]:
-
α2,3 sialyltransferase acting on Galβ1-3GalNAc
- ST3/6[Galβ1,4GlcNAc]:
-
α2,3/6 sialyltransferase acting on Galβ1-4GlcNAc
- Bn:
-
Benzyl
- PAA:
-
Polyacrylamide
- Φ-NO2 :
-
p-nitrophenol
References
Neelamegham, S., Liu, G.: Systems glycobiology: biochemical reaction networks regulating glycan structure and function. Glycobiology 21(12), 1541–1553 (2011)
Hakomori, S.: Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv. Exp. Med. Biol. 491, 369–402 (2001)
Yoshimura, M., Nishikawa, A., Ihara, Y., Taniguchi, S., Taniguchi, N.: Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc. Natl. Acad. Sci. U. S. A. 92(19), 8754–8758 (1995)
Tei, K., Kawakami-Kimura, N., Taguchi, O., Kumamoto, K., Higashiyama, S., Taniguchi, N., Toda, K., Kawata, R., Hisa, Y., Kannagi, R.: Roles of cell adhesion molecules in tumor angiogenesis induced by cotransplantation of cancer and endothelial cells to nude rats. Cancer Res. 62(21), 6289–6296 (2002)
Itzkowitz, S.H., Yuan, M., Montgomery, C.K., Kjeldsen, T., Takahashi, H.K., Bigbee, W.L., Kim, Y.S.: Expression of Tn, Sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 49(1), 197–204 (1989)
Kozlowski, E.O., Pavao, M.S.: Effect of sulfated glycosaminoglycans on tumor invasion and metastasis. Front. Biosci. 3, 1541–1551 (2011)
Stevenson, J.L., Varki, A., Borsig, L.: Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thromb. Res. 120, Supplement 2(0), S107–S111 (2007)
Potapenko, I.O., Haakensen, V.D., Lüders, T., Helland, Å., Bukholm, I., Sørlie, T., Kristensen, V.N., Lingjærde, O.C., Børresen-Dale, A.-L.: Glycan gene expression signatures in normal and malignant breast tissue; possible role in diagnosis and progression. Mol. Oncol. 4(2), 98–118 (2010)
Wander, S.A., Hennessy, B.T., Slingerland, J.M.: Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J. Clin. Invest. 121(4), 1231–1241 (2011)
Sebolt-Leopold, J.S., English, J.M.: Mechanisms of drug inhibition of signalling molecules. Nature 441(7092), 457–462 (2006)
Chandrasekaran, E.V., Xue, J., Neelamegham, S., Matta, K.L.: The pattern of glycosyl- and sulfotransferase activities in cancer cell lines: a predictor of individual cancer-associated distinct carbohydrate structures for the structural identification of signature glycans. Carbohydr. Res. 341(8), 983–994 (2006)
Zhang, H., Meng, F., Wu, S., Kreike, B., Sethi, S., Chen, W., Miller, F.R., Wu, G.: Engagement of I-branching β-1, 6-N-acetylglucosaminyltransferase 2 in breast cancer metastasis and TGF-β signaling. Cancer Res. 71(14), 4846–4856 (2011)
Barthel, S.R., Wiese, G.K., Cho, J., Opperman, M.J., Hays, D.L., Siddiqui, J., Pienta, K.J., Furie, B., Dimitroff, C.J.: Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc. Natl. Acad. Sci. U. S. A. 106(46), 19491–19496 (2009)
Carvalho, A.S., Harduin-Lepers, A., Magalhães, A., Machado, E., Mendes, N., Costa, L.T., Matthiesen, R., Almeida, R., Costa, J., Reis, C.A.: Differential expression of α-2,3-sialyltransferases and α-1,3/4-fucosyltransferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. Int. J. Biochem. Cell Biol. 42(1), 80–89 (2010)
Recchi, M.-A., Hebbar, M., Hornez, L., Harduin-Lepers, A., Peyrat, J.-P., Delannoy, P.: Multiplex reverse transcription polymerase chain reaction assessment of sialyltransferase expression in human breast cancer. Cancer Res. 58(18), 4066–4070 (1998)
Gu, Y., Mi, W., Ge, Y., Liu, H., Fan, Q., Han, C., Yang, J., Han, F., Lu, X., Yu, W.: GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res. 70(15), 6344–6351 (2010)
Seko, A., Ohkura, T., Kitamura, H., Yonezawa, S., Sato, E., Yamashita, K.: Quantitative differences in GlcNAc:β1–>3 and GlcNAc:β1–>4 galactosyltransferase activities between human colonic adenocarcinomas and normal colonic mucosa. Cancer Res. 56(15), 3468–3473 (1996)
Chandrasekaran, E., Xue, J., Piskorz, C., Locke, R., Tóth, K., Slocum, H., Matta, K.: Potential tumor markers for human gastric cancer: an elevation of glycan:sulfotransferases and a concomitant loss of α1,2-fucosyltransferase activities. J. Cancer Res. Clin. Oncol. 133(9), 599–611 (2007)
Park, S.-Y., Lee, S.-H., Kawasaki, N., Itoh, S., Kang, K., Hee Ryu, S., Hashii, N., Kim, J.-M., Kim, J.-Y., Hoe Kim, J.: α1-3/4 fucosylation at Asn 241 of β-haptoglobin is a novel marker for colon cancer: a combinatorial approach for development of glycan biomarkers. Int. J. Cancer 130(10), 2366–2376 (2012)
Fry, S.A., Afrough, B., Lomax-Browne, H.J., Timms, J.F., Velentzis, L.S., Leathem, A.J.C.: Lectin microarray profiling of metastatic breast cancers. Glycobiology 21(8), 1060–1070 (2011)
Abd Hamid, U.M., Royle, L., Saldova, R., Radcliffe, C.M., Harvey, D.J., Storr, S.J., Pardo, M., Antrobus, R., Chapman, C.J., Zitzmann, N., Robertson, J.F., Dwek, R.A., Rudd, P.M.: A strategy to reveal potential glycan markers from serum glycoproteins associated with breast cancer progression. Glycobiology 18(12), 1105–1118 (2008)
Pierce, A., Saldova, R., Abd Hamid, U.M., Abrahams, J.L., McDermott, E.W., Evoy, D., Duffy, M.J., Rudd, P.M.: Levels of specific glycans significantly distinguish lymph node-positive from lymph node-negative breast cancer patients. Glycobiology 20(10), 1283–1288 (2010)
Patil, S.A., Chandrasekaran, E.V., Matta, K.L., Parikh, A., Tzanakakis, E.S., Neelamegham, S.: Scaling down the size and increasing the throughput of glycosyltransferase assays: activity changes on stem cell differentiation. Anal. Biochem. 425(2), 135–144 (2012)
Marathe, D.D., Chandrasekaran, E.V., Lau, J.T., Matta, K.L., Neelamegham, S.: Systems-level studies of glycosyltransferase gene expression and enzyme activity that are associated with the selectin binding function of human leukocytes. FASEB J. 22(12), 4154–4167 (2008)
Chandrasekaran, E.V., Xue, J., Xia, J., Chawda, R., Piskorz, C., Locke, R.D., Neelamegham, S., Matta, K.L.: Analysis of the specificity of sialyltransferases toward mucin core 2, globo, and related structures. identification of the sialylation sequence and the effects of sulfate, fucose, methyl, and fluoro substituents of the carbohydrate chain in the biosynthesis of selectin and siglec ligands, and novel sialylation by cloned alpha2,3(O)sialyltransferase. Biochemistry 44(47), 15619–15635 (2005)
Marathe, D.D., Buffone Jr., A., Chandrasekaran, E.V., Xue, J., Locke, R.D., Nasirikenari, M., Lau, J.T., Matta, K.L., Neelamegham, S.: Fluorinated per-acetylated GalNAc metabolically alters glycan structures on leukocyte PSGL-1 and reduces cell binding to selectins. Blood 115(6), 1303–1312 (2010)
Karsten, U., Butschak, G., Cao, Y., Goletz, S., Hanisch, F.G.: A new monoclonal antibody (A78-G/A7) to the Thomsen-Friedenreich pan-tumor antigen. Hybridoma 14(1), 37–44 (1995)
Miles, D.W., Happerfield, L.C., Smith, P., Gillibrand, R., Bobrow, L.G., Gregory, W.M., Rubens, R.D.: Expression of sialyl-Tn predicts the effect of adjuvant chemotherapy in node-positive breast cancer. Br. J. Cancer 70(6), 1272–1275 (1994)
Patani, N., Jiang, W.E.N., Mokbel, K.: Prognostic utility of glycosyltransferase expression in breast cancer. Cancer Genomics Proteomics 5(6), 333–340 (2008)
Sewell, R., Bäckström, M., Dalziel, M., Gschmeissner, S., Karlsson, H., Noll, T., Gätgens, J., Clausen, H., Hansson, G.C., Burchell, J., Taylor-Papadimitriou, J.: The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J. Biol. Chem. 281(6), 3586–3594 (2006)
Lloyd, K.O., Burchell, J., Kudryashov, V., Yin, B.W.T., Taylor-Papadimitriou, J.: Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. J. Biol. Chem. 271(52), 33325–33334 (1996)
Brockhausen, I., Yang, J.-M., Burchell, J., Whitehouse, C., Taylor-Papadimitriou, J.: Mechanisms underlying aberrant glycosylation of MUC1 mucin in breast cancer cells. Eur. J. Biochem. 233(2), 607–617 (1995)
Storr, S.J., Royle, L., Chapman, C.J., Hamid, U.M.A., Robertson, J.F., Murray, A., Dwek, R.A., Rudd, P.M.: The O-linked glycosylation of secretory/shed MUC1 from an advanced breast cancer patient’s serum. Glycobiology 18(6), 456–462 (2008)
Burchell, J., Poulsom, R., Hanby, A., Whitehouse, C., Cooper, L., Clausen, H., Miles, D., Taylor-Papadimitriou, J.: An α2,3 sialyltransferase (ST3Gal I) is elevated in primary breast carcinomas. Glycobiology 9(12), 1307–1311 (1999)
Picco, G., Julien, S., Brockhausen, I., Beatson, R., Antonopoulos, A., Haslam, S., Mandel, U., Dell, A., Pinder, S., Taylor-Papadimitriou, J., Burchell, J.: Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology 20(10), 1241–1250 (2010)
Muller, S., Hanisch, F.-G.: Recombinant MUC1 probe authentically reflects cell-specific O-glycosylation profiles of endogenous breast cancer mucin. J. Biol. Chem. 277(29), 26103–26112 (2002)
Engelmann, K., Kinlough, C.L., Muller, S., Razawi, H., Baldus, S.E., Hughey, R.P., Hanisch, F.-G.: Transmembrane and secreted MUC1 probes show trafficking-dependent changes in O-glycan core profiles. Glycobiology 15(11), 1111–1124 (2005)
Acknowledgments
This work was supported by the National Institutes of Health [grant HL63014(SN), CA121294 (KLM) and HL103411(SN)] and Department of Defense [grant DODPC050420 (KLM)]].
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1514 kb)
Rights and permissions
About this article
Cite this article
Patil, S.A., Bshara, W., Morrison, C. et al. Overexpression of α2,3sialyl T-antigen in breast cancer determined by miniaturized glycosyltransferase assays and confirmed using tissue microarray immunohistochemical analysis. Glycoconj J 31, 509–521 (2014). https://doi.org/10.1007/s10719-014-9548-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-014-9548-4