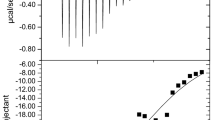
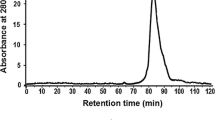
Abstract
T/Tn specificity of Artocarpus lakoocha agglutinin (ALA), isolated from the seeds of A. lakoocha (Moraceae) fruit and a heterodimer (16 kD and 12 kD) of molecular mass 28 kD, was further confirmed by SPR analysis using T/Tn glycan containing mammalian glycoproteins. N-terminal amino acid sequence analysis of ALA showed homology at 15, 19–21, 24–27, and 29 residues with other lectin members of Moraceae family viz., Artocarpus integrifolia (jacalin) lectin, Artocarpus hirsuta lectin, and Maclura pomifera agglutinin. It is mitogenic to human PBMC and the maximum proliferation was observed at 1 ng/ml. It showed an antiproliferative effect on leukemic cells, with the highest effect toward Jurkat cells (IC50 13.15 ng/ml). Synthesized CdS quantum dot-ALA nanoconjugate was employed to detect the expression of T/Tn glycans on Jurkat, U937, and K562 leukemic cells surfaces as well as normal lymphocytes by fluorescence microscopy. No green fluorescence was observed with normal lymphocytes indicating that T/Tn determinants, which are recognized as human tumor associated structures were cryptic on normal lymphocyte surfaces, whereas intense green fluorescent dots appeared during imaging of leukemic cells, where such determinants were present in unmasked form. The above results indicated that QD-ALA nanoconjugate is an efficient fluorescent marker for identification of leukemic cell lines that gives rise to high quality images.









Similar content being viewed by others
Abbreviations
- ALA:
-
Artocarpus lakoocha agglutinin
- BSM:
-
Bovine submandibular gland mucin
- CdS:
-
Cadmium sulphide
- ELLSA:
-
Enzyme - linked lectinsorbent assay
- ESI-MS:
-
Electron spray ionization mass spectrometry
- FPLC:
-
Fast protein liquid chromatography
- HBS:
-
Hepes buffered saline
- HEPES:
-
N-(2-hydoxyethyl) piperizine-N′-(2-hydroxypropane sulfonic acid)
- HSM:
-
Hamster submaxillary mucin
- PBMC:
-
Peripheral blood mononuclear cells
- PVDF:
-
Polyvinylidene difluoride
- Q-ToF:
-
Quadrupole-time of flight
- QD:
-
Quantum dot
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- 2 ME:
-
2-Mercaptoethanol
References
Dixon, H.B.F.: Defining a lectin. Nature 292, 192 (1981)
Sharon, N., Lis, H.: Lectins as cell recognition molecules. Science 246, 227–234 (1989)
Hakomori, S.: Glycosylation defining cancer malignancy: New wine in an old bottle. Proc. Natl. Acad. Sci. USA. 99, 10231–10233 (2002)
Kim, Y.J., Varki, A.: Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj. J. 14, 569–576 (1997)
Strauchen, J.A.: Lectin receptor as markers of lymphoid cells. II Reed-Sternberg cells share lectin-binding properties of monocyte macrophages. Am. J. Pathol. 116, 370–376 (1984)
Cook, D.B., Bustamam, A.A., Brotherick, I., Shenton, B.K., Self, C.H.: Lectin ELISA for the c-erb-B2 tumor marker protein p185 in patients with breast cancer and controls. Clin. Chem. 45, 292–295 (1999)
Kamoto, T., Satomura, S., Yoshiki, T., Okada, Y., Henmi, F., Nishiyama, H., Kobayashi, T., Terai, A., Habuchi, T., Ogawa, O.: Lectin-reactive α-fetoprotein (AFP-L3%) curability and prediction of clinical course after treatment of non-seminomatous germ cell tumors. Jpn. J. Clin. Oncol. 32, 472–476 (2002)
Comunale, M.A., Lowman, M., Long, R.E., Krakover, J., Philip, R., Seeholzer, S., Evans, A.A., Hann, H.W.L., Block, T.M., Mehta, A.S.: Proteomic analysis of serum associated fucosylated glycoproteins in the development of primary hepatocellular carcinoma. J. Proteom. Res. 5, 308–315 (2006)
Lingerfelt, B.M., Mattoussi, H., Goldman, E.R., Mauro, J.M., Anderson, G.P.: Preparation of quantum dot-biotin conjugates and their use in immunochromatography assay. Anal. Chem. 75, 4043–4049 (2003)
Kaul, Z., Yaguchi, T., Kaul, S.C., Hirano, T., Wadhwa, R., Taira, K.: Mortalin imaging in normal and cancer cells with quantum dot immuno-conjugates. Cell Res. 13, 503–507 (2003)
Goldman, E.R., Clapp, A.R., Anderson, G.P., Uyeda, H.T., Mauro, J.M., Medintz, I.L., Mattoussi, H.: Multiplexed toxin analysis using four colors of quantum dot fluororeagents. Anal. Chem. 76, 684–688 (2004)
Chowdhury, S., Ahmed, H., Chatterjee, B.P.: Purification and characterization of an α-D-galactopyranosyl binding lectin from Artocarpus lakoocha seeds. Carbohydr. Res. 159, 137–148 (1987)
Chatterjee, B.P., Ahmed, H., Chowdhury, S.: Further characterization of Artocarpus lakoocha lectin (Artocarpin) purified using rivanol. Carbohydr. Res. 180, 97–110 (1988)
Chowdhury, S., Chatterjee, B.P.: Artocarpin–galactomannan interaction: Characterization of combining site of artocarpin. Phytochemistry 32, 243–249 (1993)
Singh, T., Chatterjee, U., Wu, J.H., Chatterjee, B.P., Wu, A.M.: Carbohydrate recognition factors of a Ta (Gal β1→ 3 GalNAC α 1 →Ser/Thr) and Tn (GalNAc α 1 →Ser/Thr) specific lectin isolated from the seeds of Artocarpus lakoocha. Glycobiology 15, 67–78 (2005)
Wu, A.M., Pigman, W.: Preparation and characterization of armadillo submandibular glycoproteins. Biochem. J. 161, 37–47 (1977)
Duk, M., Lisowska, E., Wu, J.H., Wu, A.M.: The biotin/avidin mediated microtiter plate lectin assay with the use of chemically modified glycoprotein ligand. Anal. Biochem. 221, 266–272 (1994)
Teichberg, V.I., Aberdam, D., Erez, U., Pinelli, E.: Affinity-repulsion chromatography, principle and application to lectins. J. Biol. Chem. 263, 14086–14092 (1988)
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976)
Bhowal, J., Guha, A.K., Chatterjee, B.P.: Purification and molecular characterization of a sialic acid specific lectin from the phytopathogenic fungus Macrophomina phaseolina. Carbohydr. Res. 340, 1973–1982 (2005)
Reisfled, R.A., Lewis, U.S., Williams, D.E.: Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature 195, 281–283 (1962)
Laemmli, U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277, 680–685 (1970)
Towbin, H., Staehelin, T., Gordon, J.: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proct. Natl. Acad. Sci. USA. 76, 4350–4354 (1979)
Edman, P., Begg, G.: A protein sequencer. Eur. J. Biochem. 1, 80–91 (1967)
Szabo, A., Stolz, L., Granzow, R.: Surface plasmon resonance and its use in biomolecular interaction analysis (BIA). Curr. Opin. Struct. Biol. 5, 699–705 (1995)
Bhattacharyya, I., Mandal, C., Chowdhury, M.: Functional heterogeneity of sialic acid binding agglutinin of rat uteri towards in vitro lymphocyte transformation. Am. J. Reprod. Immunl. 20, 81–86 (1989)
Mandal, C., Chowdhury, M.: The polyclonal activation of lymphocytes and T cell mitogenicity by a unique sialic-acid-binding lectin from the hemolymph of Achatina fulica snail. Immunopharmacology 20, 63–72 (1990)
Wong, J.H., Ng, T.B.: Isolation and characterization of a glucose/mannose/rahmnose specific lectinfrom the knife bean Canavalia gladiata. Arch. Biochem. Biophys. 439, 91–98 (2005)
Ngai, P.H.K., Ng, T.B.: A mushroom (Ganoderma capense) lectin with spectacular thermostability, potent mitogenic activity on splenocytes, and antiproliferative activity toward tumor cells. Biochem. Biophys. Res. Com. 314, 988–993 (2004)
Chatterjee, B.P., Ahmed, H.: Lectins from plants and animals: carbohydrate specificity, unity in diversity and diversity in unity. Biochem. Arch. 14, 1–5 (1998)
Namjuntra, P., Muanwongyathi, P., Chulavatnatol, R.: A sperm agglutinating lectin from seeds of jack fruit (Artocarpus heterophyllus). Biochem. Biophys. Res. Commun. 128, 833–839 (1985)
Chei, W.G., Hounsell, E.F., Cashmore, G.C., Rosankie-wicz, J.R., Bauer, C.J., Feeney, J., Feizi, T., Lawson, A.M.: Neutral oligosaccharides of bovine submaxillary mucin. A combined mass spectrometry and 1H-NMR study. Eur. J. Biochem. 203, 257–268 (1992)
Podbielska, M., Fredriksson, S.A., Nilsson, B., Lisowaska, E., Krotkeiewski, H.: ABH blood group antigens in O-glycans of human glycophorin A. Arch. Biochem. Biophys. 429, 145–153 (2004)
Nilsson, B., Norden, N.E., Svensson, S.: Structural studies on the carbohydrate portion of fetuin. J. Biol.Chem. 254, 4545–4553 (1979)
Springer, G.: T and Tn pancarcinoma markers: autoantigenic adhesion molecules in pathogenesis, pre-biopsy carcinoma detection and long-term breast-carcinoma immunotherapy. Crit. Rev. Oncogen. 6, 57–85 (1995)
Springer, G.: Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis and immunotherapy. J. Mol. Med. 75, 594–602 (1997)
Singh, J., Singh, J., Kamboj, S.S.: A novel mitogenic and antiproliferative lectin from a wild cobra lily, Arisaema flavum. Biochem. Biophys. Res. Commun. 318, 1057–1065 (2004)
Wang, H.X., Ng, T.B., Liu, W.K., Ooi, V.E.C., Chang, S.T.: Isolation and characterization of two distinct lectins with antiproliferative activity from the cultured mycelium of the edible mushroom Tricholoma mangolicum. Int. Peptide Protein Res. 46, 508–513 (1995)
Xiaochao, X.U., Chuanfang, W.U., Chao, L.I.U., Yongting, L.U.O., Jian, L.I., Xinping, Z.H.A.O., Van Damme, E., Jinku, B.: Purification and characterization of a mannose-binding lectin from the rhizomes of Aspidistra elatior Blume with antiproliferative activity. Acta. Biochim. Biophys. Sinica 39, 507–519 (2007)
Wong, J.J., Ng, T.B.: Purification of a trypsin-stable lectin with antiproliferative and HIV-1 reverse transcriptase inhibitory activity. Biochem. Biophys. Res. Commun. 301, 545–550 (2003)
Wang, H., Ng, T.B., Ooi, V.E., Liu, W.K.: Effects of lectins with different carbohydrate binding specificities on hepatoma, choriocarcinoma, melanoma and osteosarcoma cell lines. Int. J. Biochem. Cell Biol. 32, 365–372 (2000)
Acknowledgements
We sincerely thank Dr. Sujata Sharma of Department of Biophysics, All India Institutes of Medical Sciences, New Delhi for performing amino acid sequence analysis. The assistance of Dr. Syamal Roy of Indian Institute of Chemical Biology, Kolkata and Mr. Gautam Mondal of West Bengal University of Technology, Kolkata is gratefully acknowledged. This study was financially supported by a research grant (BT/PR4462/BRB/10/1350/2003) of the Department of Biotechnology, Government of India, New Delhi to B.P.C.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chatterjee, U., Bose, P.P., Dey, S. et al. Antiproliferative effect of T/Tn specific Artocarpus lakoocha agglutinin (ALA) on human leukemic cells (Jurkat, U937, K562) and their imaging by QD-ALA nanoconjugate. Glycoconj J 25, 741–752 (2008). https://doi.org/10.1007/s10719-008-9134-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-008-9134-8