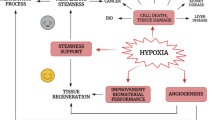
Abstract
During tumor growth and invasion, the endothelial cells from a relatively quiescent endothelium start proliferating. The exact mechanism of switching to a new angiogenic phenotype is currently unknown. We have examined the role of intracellular cAMP in this process. When a non-transformed capillary endothelial cell line was treated with 2 mM 8Br-cAMP, cell proliferation was enhanced by ∼70%. Cellular morphology indicated enhanced mitosis after 32–40 h with almost one-half of the cell population in the S phase. Bcl-2 expression and caspase-3, -8, and -9 activity remained unaffected. A significant increase in the Glc3Man9GlcNAc2-PP-Dol biosynthesis and turnover, Factor VIIIC N-glycosylation, and cell surface expression of N-glycans was observed in cells treated with 8Br-cAMP. Dol-P-Man synthase activity in the endoplasmic reticulum membranes also increased. A 1.4–1.6-fold increase in HSP-70 and HSP-90 expression was also observed in 8Br-cAMP treated cells. On the other hand, the expression of GRP-78/Bip was 2.3-fold higher compared to that of GRP-94 in control cells, but after 8Br-cAMP treatment for 32 h, it was reduced by 3-fold. GRP-78/Bip expression in untreated cells was 1.2–1.5-fold higher when compared with HSP-70 and HSP-90, whereas that of the GRP-94 was 1.5–1.8-fold lower. After 8Br-cAMP treatment, GRP-78/Bip expression was reduced 4.5–4.8-fold, but the GRP-94 was reduced by 1.5–1.6-fold only. Upon comparison, a 2.9-fold down-regulation of GRP-78/Bip was observed compared to GRP-94. We, therefore, conclude that a high level of Glc3Man9GlcNAc2-PP-Dol, resulting from 8Br-cAMP stimulation up-regulated HSP-70 expression and down-regulated that of the GRP-78/Bip, maintained adequate protein folding, and reduced endoplasmic reticulum stress. As a result capillary endothelial cell proliferation was induced.
Similar content being viewed by others
Abbreviations
- EMEM:
-
minimal essential medium with Earle’s salt
- DMEM:
-
Dulbeccos’ minimal essential medium
- NP-40:
-
Nonidet P-40
- SDS:
-
sodium dodecyl sulfate
- PBS:
-
phosphate-buffer-saline
- PAGE:
-
polyacrylamide gel electrophoresis
- HRP:
-
horseradish peroxidase
- Dol-P:
-
dolicylmonophosphate
- DMSO:
-
dimethylsolfoxide
- Con A:
-
concanavalin A
- WGA:
-
wheat germ agglutinin
- cAMP:
-
adenosine 3′, 5′-cyclic monophosphate
- PKA:
-
cAMP-dependent protein kinase
- ER:
-
endoplasmic reticulum
- LLO:
-
Glc3Man9GlcNAc2-PP-Dol
- Dol-P-Man:
-
dolichol-P-Mannose
- Dol-P-Glc:
-
dolichol-P-glucose
- GPT:
-
UDP-GlcNAc-dolichol-P GlcNAc-1-P transferase
- DPMS:
-
Dol-P-Man synthase
- UPR:
-
unfolded protein response
References
Conway, E.M., Collen, D., Carmeliet, P.: Molecular mechanisms of blood vessel growth. Cardiovascular Res. 49, 507–521 (2001)
Hamada, J., Cavanaugh, P.G., Lotan, O.: Seperable growth and migration factors for large-cell lymphoma cells secreted by microvascular endothelial cells derived from target organs for metastasiss. Br. J. Cancer Res. 66, 349–354 (1992)
Folkman, J.: Angiogenesis and breast cancer. J. Clin. Oncol. 12, 441–443 (1994)
Stuart, S.B., Smith, N., Brunner, N., Harris, A.L.: High level of uPA and PA-1 are associated with highly angiogenic breast carcinoma. J. Pathol. 170, 388a (1993)
Gross, J.L., Moscatalli, D., Jaffe, E.A., Rifkin, D.: Plasminogen activator and collagenase production by cultured capillary endothelial cells. J. Cell Biol. 95, 974–981 (1982)
Vartanuan, R., Weidner, N.: Correlation of intratumoral endothelial cell proliferation with microvessel density (tumor angiogenesis) and tumor-cell proliferation in breast carcinoma. Am. J. Pathol. 144, 1188–1194 (1994)
Dvorak, H.F., Tumors: wounds that do not heal. Similarities between tumor generation and wound healing. N. Engl. J. Med. 315, 1650–1659 (1986)
Ellgaard, L., Molinari, M., Helenius, A.: Setting the standards: quality control in the secretory pathway. Science 286, 1882–1888 (1999)
Kornfeld, R., Kornfeld, S.: Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 54, 631–664 (1985)
Chapman, A., Trowbridge, I.S., Hyman, R., Kornfeld, S.: Structure of the lipid-linked oligosaccharides that accumulate in class E Thy-1-negative mutant lymphoma. Cell 17, 509–515 (1979)
Banerjee, D.K., Scher, M.G., Waechter, C.J., Amphomycin: Effect of the lipopeptide antibiotic on the glycosylation and extraction of dolichyl monophosphate in calf brain membranes. Biochemistry 20, 1561–1568 (1981)
Lehrman, M.A.: Biosynthesis of N-acetylglucosamine-P-P-dolichol, the committed step of asparagine-linked oligosaccharide assembly. Glycobiology 1, 553–562 (1991)
Kean, E.L., Rush, J.S., Waechter, C.J.: Activation of GlcNAc-P-P-dolichol synthesis by mannosylphosphoryldolichol is stereospecific and requires a saturated alpha-isoprene unit. Biochemistry 33, 10508–10512 (1994)
Kean, E.L., Wei, Z., Anderson, V.E., Zhang, N., Sayre, L.M.: Regulation of the biosynthesis of N-acetylglucosaminyl- pyrophosphoryldolichol, feedback and product inhibition. J. Biol. Chem. 274, 34072–34082 (1999)
Banerjee, D.K., Kousvelari, E.E., Baum, B.J., cAMP-mediated protein phosphorylation of microsomal membranes increases mannosylphospho dolichol synthase activity. Proc. Natl. Acad. Sci. (USA) 84, 6389–6393 (1987)
Banerjee, D.K., Aponte, E., DaSilva, J.J.: Low expression of lipid-linked oligosaccharide due to a functionally altered Dol-P-Man synthase reduces protein glycosylation in cAMP-dependent protein kinase deficient Chinese hamster ovary cells. Glyconjugate. J. 21, 479–486 (2004)
Banerjee, D.K., Carrasquillo, E.A., Hughy, P., Schutzbach, J.S., Martínez, J.A., Baksi, K., In. vitro. phosphorylation by cAMP-dependent protein kinase up-regulates recombinant Saccharomyces cerevisiae mannosylphosphodolichol. J. Biol. Chem. 280, 4174–4181 (2005)
Banerjee, D.K.: Microenvironment of endothelial cell growth and regulation of protein N-glycosylation. Indian. J. Biochem. Biophys. 25, 8–13 (1988)
Oliveira, C.M., Banerjee, D.K.: Role of extracellular signaling on endothelial cell proliferation and protein N-glycosylation. J. Cellular Physiol. 144, 467–472 (1990)
Banerjee, D.K., Vendrell-Ramos, M.: Is asparagine-linked protein glycosylation an obligatory requirement for angiogenesis? Indian J. Biochem. Biophys. 30, 389–394 (1993)
Tavárez-Pagán, J.J., Oliveira, C.M., Banerjee, D.K.: Insulin up-regulates a Glc3Man9GlcNAc2-PP-Dol pool in capillary endothelial cells not essential for angiogenesis. Glycoconjugate J. 20, 179–188 (2004)
Banerjee, D.K.: Regulation of mannosylphosphoryl dolichol synthase activity by cAMP-dependent protein phosphorylation. In Highlights of Modern Biochemistry, edited by Kotyk, A., Skoda, J., Paces, V., Kostka, V (VSP International Science Publishers, Zeist, The Neetherlands, 1989), p. 379
Das, S.K., Mukherjee, S., Banerjee, D.K.: Beta-adrenoreceptors of multiple affinities in a clonal capillary endothelial cell line and its functional implications. Molec. Cellular Biochem. 140, 49–54 (1994)
Banerjee, D.K., Ornberg, R.L., Youdim, M.B., Heldman, E., Pollard, H.B.: Endothelial cells from bovine adrenal medulla develop capillary-like growth patterns in culture. Proc. Natl. Acad. Sci. (USA) 82, 4702–4706 (1985)
Martínez, J.A.: Torres-Negrón, I., Amigó, L.A, Banerjee, D.K.: Expression of Glc3Man9GlcNAc2-PP-Dol is a prerequisite for capillary endothelial cell proliferation. Cellular Molec. Biochem. 45, 137–152 (1999)
Krishan, A.: Rapid flow cytometric analysis of mammalian cell cycle by propidium iodide staining. J. Cell Biol. 66, 188–195 (1975)
Vindelov, L.L.: Flow cytometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. Virchows Arch. (B) 24, 227–231 (1977)
Banerjee, D.K., Tavárez, J.J., Oliveira, C.M.: Expression of blood clotting factor VIII:C gene in capillary endothelial cells. FEBS Letts. 306, 33–37 (1992)
Bradford, M.M.: A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976)
Banerjee, D.K.: Amphomycin inhibits mannosylphosphoryldolichol synthesis by forming a complex with dolichylmonophosphate. J. Biol. Chem. 264, 2024–2028 (1989)
Kluck, R.M., Martin, S.J., Hoffman, B.M., Zhou, J.S., Green, D.R., Newmeyer, D.D.: Cytochrome c activation of CPP32-like proteolysis plays a critical role in a Xenopus cell-free apoptosis system. EMBO J. 16, 4639–4649 (1997)
Morishima, N., Nakanishi, K., Takenoouchi, H., Shibata, T., Yasuhiko, Y.: An endoplasmic reticulum stress-specific caspase cascade in apoptosis. J. Biol. Chem. 277, 34287–34294 (1977)
Thornberry, N.A., Lazebnik, Y.: Caspases: enemies within. Science 281, 1312–1316 (1998)
Cryns, V.L., Yuan, J.: Proteases to die for. Genes. Dev. 12, 1551–1570 (1998)
Harding, H.P., Calfon, M., Urano, F., Novoa, I., Ron, D.: Transcriptional and translational control in the Mammalian unfolded protein response. Annu. Rev. Cell Dev. Biol. 18, 575–599 (2002)
Buku, B., Horwich, A.L.: The Hsp, 70 and Hsp, 60 chaperones machines. Cell 92, 351–366 (1998)
Pearl, L.H., Prodromon, C.: Structure and in vivo function of HSP-90. Curr. Opin. Struct. Biol. 10, 46–51 (2000)
Taylor, S.S., Buechler, J.A., Yonemoto, W.: cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu. Rev. Biochem. 59, 971–1005 (1990)
Conti, M., Richter, W., Mehats, C., Livera, G., Park J-Y, Jin, C.: Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J. Biol. Chem. 276, 5493–5496 (2003)
Rubin, C.S.: A kinase anchor proteins and the intracellular targeting of signals carried by cyclic AMP. Biochim. Biophys. Acta. 1224, 467–479 (1994)
Flockhart, D.A., Corbin, J.D.: Regulatory mechanisms in the control of protein kinases. Crit. Rev. Biochem. 12, 133–186 (1982)
Gottesman, M.M.: Genetics of cAMP-dependent protein kinases. In Molecular Cell Genetics, Gottesman MM (ed), John Wiley and Sons Inc., New York, USA, pp. 711–743 (1985)
Krebs, E.G., Beavo, J.A.: Phosphorylation-dephosphorylation of enzymes. Annu. Rev. Biochem. 48, 923–959 (1979)
Kuo, J.F., Greengard, P.: Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrance (1969) of 3′,5′-monophosphate-dependent protein kinases in various tissues and phyla of the animal kingdom. Proc. Natl. Acad. Sci. (USA) 64, 1349–1355 (1969)
Lohmann, S.M., Walter, U.: Regulation of the cellular and subcellular concentrations and distribution of cyclic nucelotide-dependent protein kinases. Adv. Cyclic Nucleotide Protein Phosphorylation Res., 18, 63–177 (1984)
Walsh, D.A., Perkins, J.P., Krebs, E.G.: An adenosine 3′,5′-monophosphate dependent protein kinase from rabbit skeletal muscle. J. Biol. Chem. 234, 3763–3774 (1968)
Morimato, R.I., Tissieres, A., Georgopoulos, C.: The biology of heat shock proteins and molecular chaperones. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press) (1994)
Hartl, F.U.: Molecular chaperones in cellular protein folding. Nature 381, 571–580 (1996)
Flynn, G.C., Chappell, T.G., Rothman, J.E.: Peptide-binding specificity of the molecular chaperone Bip. Nature 353, 726–730 (1991)
Rüdiger, S., Germeroth, L., Bukau, B.: Interaction of Hsp 70 chaperones with substrates. Nature Struct. Biol. 4, 342–349 (1997)
Szabo, A., Korzun, R., Hartl, F.U., Flanagan, J.: A zinc finger-like domain of the molecular chaperone DnaJ is involved in binding to determined protein substrates. EMBO J. 15, 408–417 (1996)
Buchberger, A., Theyssen, H., Schröder, H., McCarty, J.S., Virgallita, G., Milkereit, P., Reinstein, J., Buku, B.: Nucleotide-induced conformational changes in the ATPase and substrate binding domains of the DnaK chaperone provide evidence for interdomain communication. J. Biol. Chem. 270, 16903–16910 (1995)
Hendrick, J.P., Hartl, F.U.: Molecular chaperone functions of heat-shock proteins. Annu. Rev. Biochem. 62, 349–384 (1993)
Landry, S.J., Gierasch, L.M.: Polypeptide interactions with molecular chaperones and their relationship to in vivo protein folding. Annu. Rev. Biophys. Biomolec. Struct. 23, 645–669 (1994)
Randall, L.L., Hardy, S.J.S.: High selectivity with low specificity: how SecB has solved the paradox of chaperone binding. Trends Biochem. Sci. 20, 65–69 (1995)
Neuport, W., Hartl, F.U., Craig, E.A., Pfanner, N.: How do polypeptides cross the mitochondrial membranes? Cell 63, 447–450 (1990)
Simon, S.M., Peskin, C.S., Oster, G.F.: What drives the translocation of proteins? Proc. Natl. Acad. Sci. (USA) 89, 3770–3774 (1992)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez, J.A., Tavárez, J.J., Oliveira, C.M. et al. Potentiation of angiogenic switch in capillary endothelial cells by cAMP: A cross-talk between up-regulated LLO biosynthesis and the HSP-70 expression. Glycoconj J 23, 209–220 (2006). https://doi.org/10.1007/s10719-006-7926-2
Issue Date:
DOI: https://doi.org/10.1007/s10719-006-7926-2