Abstract
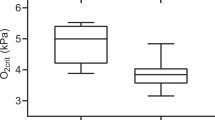
Regulation of arterial partial pressure of O2 (PaO2) in Atlantic salmon (Salmo salar) was investigated during resting conditions in normoxic and hyperoxic water. Dorsal aorta cannulated adult Atlantic salmon (1.2–1.6 kg, n = 8) were exposed to 2 week sequential periods of normoxia [16.7 ± 1.1 kPa (mean ± SD)] and hyperoxia (34.1 ± 4.9 kPa) in individual tanks containing seawater (33.7 ± 0.2 ppt) at stable temperature conditions (8.7 ± 0.7°C) and a light regime of L:D = 12:12. Tank design and sampling procedures were optimized to provide suitable shelter and current for the fish, and to allow repeated, undisturbed sampling of blood from free-swimming fish. Fish were sampled regularly through the experimental period. PwO2, PaO2, blood ion composition (Na+, K+, Cl−), acid–base status (pH, PCO2, HCO3 −), haematocrit and glucose were measured. The most frequently observed PaO2 values were in the range of 60–80% of PwO2, both during normoxia and hyperoxia, and PaO2 values were significantly lower during normoxia than during hyperoxia. Blood pH, PCO2 and HCO3 − were significantly elevated during hyperoxia, while, Na+, Cl− and Hct were significantly lower. K+ and glucose showed no significant differences. This study demonstrates a lack PaO2 regulation in Atlantic salmon to low partial pressures, in contrast to previous reports for many aquatic gill breathing animals. Both during normoxia and hyperoxia, PaO2 reflects PwO2, and alterations in external PO2 consequently result in proportional arterial PO2 changes. Physiological adaptation to hyperoxia, as illustrated by changes in several blood parameters, does not include down-regulation of PaO2 in Atlantic salmon. The lack of PaO2 regulation may make Atlantic salmon vulnerable to the oxidative stress caused by increased free radical formation in hyperoxic conditions.



Similar content being viewed by others
References
Angersbach D (1978) Oxygen transport in the blood of the tarantula Eurypelma californicum: PO2 and pH during rest, activity and recovery. J Comp Physiol B 123:113–125
Aota S, Randall DJ (2004) The effect of exogenous catecholamines on the ventilatory and cardiac responses of normoxic and hyperoxic rainbow trout, Oncorhynchus mykiss. J Comp Physiol Biochem B 163:138–146
Bjerkeng B, Berge GM (2000) Apparent digestibility coefficients and accumulation of astaxanthin E/Zisomers in Atlantic salmon (Salmo salar L.) and Atlantic halibut (Hippoglossus hippoglossus L.). Comp Biochem Physiol B 27(3):423–432
Brauner CJ (1999) The effect of diet and short duration hyperoxia exposure on seawater transfer in coho salmon smolts (Oncorhynchus kisutch). Aquaculture 177:257–265
Brauner CJ, Seidelin M, Madsen SS, Jensen FB (2000) Effects of fresh water hyperoxia and hypercapnia exposures and their influences on subsequent seawater transfer in Atlantic salmon (Salmo salar) smolts. Can J Fish Aquat Sci 57:2054–2064
Bushnell PG, Lutz PL, Steffensen JF, Oikarj A, Gruber SH (1982) Increases in arterial blood oxygen during exercise in the lemon shark (Negaprion brevirostris). J Comp Physiol 147:41–47
Caldwell CA, Hinshaw J (1994) Physiological and hematological responses in rainbow-trout subjected to supplemental dissolved-oxygen in fish culture. Aquaculture 126:183–193
Dabrowski K, Lee K-J, Guz L, Verlhac V, Gabaudan J (2004) Effects of dietary ascorbic acid on oxygen stress (hypoxia or hyperoxia), growth and tissue vitamin concentrations in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 233:383–392
Dejours P (1981) Principles of comparative respiratory physiology, 2nd edn. Amstedam, North Holland, 153 pp
Djordjevic B, Kristensen T, Øverli Ø, Rosseland BO, Kiessling A (2009) Effect of nutritional status and sampling intensity on recovery after dorsal aorta cannulation in free-swimming Atlantc salmon (Salmo salar L.). Fish Physiol Biochem. doi:10.1007/s10695-009-9362-2
Edsall DA, Smith CE (1990) Performance of rainbow trout and Snake river cutthroat trout reared in oxygen supersaturated water. Aquaculture 90:251–259
Eliason EJ, Kiessling A, Karlsson A, Djordjevic B, Farrell AP (2007) Validation of the hepatic portal vein cannulation technique using Atlantic salmon Salmo salar L. J Fish Biol 71:290–297
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Fandrey J, Frede S, Jelkmann W (1994) Role of hydrogen peroxide in hypoxia-induced erythropoietin production. Biochem J 303:507–510
Farrelly C, Greenaway P (1994) Gas exchange through the lungs and gills in air-breathing crabs. J Exp Biol 187:113–130
Forgue J, Burtin B, Massabuau J-C (1989) Maintenance of oxygen consumption in resting Silurus glanis at different levels of ambient oxygenation. J Exp Biol 143:305–319
Gilmour KM, Perry SF (1994) The effects of hypoxia, hyperoxia or hypercapnia on the acid-base disequilibrium in the arterial blood of rainbow trout. J Exp Biol 192:269–284
Hamre K, Kolås K, Sandnes K, Julshamn K, Kiessling A (2001) Feed intake and absorption of lipid oxidation products in Atlantic salmon (Salmo salar) fed diets coated with oxidised fish oil. Fish Physiol Biochem 25:209–219
Harrenstien LA, Tornquist SJ, Miller-Morgan TJ, Fodness BG, Clifford KE (2005) Evaluation of a point-of-care blood analyzer and determination of reference ranges for blood parameters in rockfish. J Am Vet Med Assoc 226:255–265
Hetz SK, Bradley TJ (2005) Insects breathe discontinuously to avoid oxygen toxicity. Nature 433:516–519
Hosfeld CD, Engevik A, Mollan T, Lunde TM, Waagbø R, Olsen AB, Breck O, Stefansson S, Fivelstad S (2008) Long-term separate and combined effects of environmental hypercapnia and hyperoxia in Atlantic salmon (Salmo salar L.) smolts. Aquaculture 280:146–153
Jacobs E, Vadasdi E, Sarkozi L, Coman N (1993) Analytical evaluation of i-STAT portable clinical analyzer and use by nonlaboratory health-care professionals. Clin Chem 39:1069–1074
Kiessling A, Dosanjh B, Higgs D, Deacon G, Rowshandeli N (1995) Dorsal aorta cannulation; a method to monitor changes in blood levels of astaxanthin in voluntarily feeding Atlantic salmon. Aquac Nutr 1:43–50
Kiessling A, Olsen RE, Buttle L (2003) Given the same dietary inclusion Atlantic salmon, Salmo salar (L.) display higher blood levels of canthaxanthin than astaxanthin. Aquac Nutr 9:253–262
Kiessling A, Johansson D, Zahl IH, Samuelsen OB (2009) Pharmacokinetics, plasma cortisol and effectiveness of benzocaine, MS-222 and isoeugenol measured in individual dorsal aorta-cannulated Atlantic salmon (Salmo salar) following bath administration. Aquaculture 286:301–308
Kreiberg H, Powell J (1991) Metomidate sedation reduces holding stress in chinook salmon. World Aquac 22:58–59
Leef MJ, Harris JO, Powell MD (2007) The respiratory effects of chloramine-T exposure in seawater acclimated and amoebic gill disease-affected Atlantic salmon Salmo salar L. Aquaculture 266:77–86
Liepelt A, Karbe L, Westendo J (1995) Induction of DNA strand breaks in rainbow trout, Oncorhynchus mykiss, under hypoxic and hyperoxic conditions. Aquat Toxicol 33:177–181
Lushchak VI, Bagnyukova TV (2006) Effects of different environmental oxygen levels on free radical processes in fish. Comp Biochem Physiol B 144(3):283–289
Lygren B, Hamre K, Waagbø R (2000) Effect of induced hyperoxia on the antioxidant status of Atlantic salmon Salmo salar L. fed three different levels of dietary vitamin E. Aquac Res 31:401–407
Massabuau J-C (2001) From a low arterial to low tissue oxygenation strategy, an evolutionary strategy. Respir Physiol 128:249–261
Massabuau J-C (2003) Primitive, and protective, our cellular oxygenation status? Mech Ageing Dev 124:857–863
McKenzie DJ, Wong S, Randall DJ, Egginton S, Taylor EW, Farrell AP (2004) The effects of sustained exercise and hypoxia upon oxygen tensions in the red muscle of rainbow trout. J Exp Biol 207:3629–3637
Olsen RE, Kiessling A, Milley JE, Ross NW, Lall SP (2005) Lipid source, not bile acids, affects absorption of astaxanthin in Atlantic salmon, Salmo salar L. Aquaculture 250:804–812
Olsvik PA, Kristensen T, Waagbø R, Rosseland BO, Tollefsen KE, Bæverfjord G, Berntssen MHG (2005) SOD, CAT and GSH-Px mRNA expression and lipid peroxidative stress in liver of Atlantic salmon Salmo salar exposed to hyperoxic conditions during smoltification. Comp Biochem Physiol C 141(3):314–323
Olsvik PA, Kristensen T, Waagbø R, Tollefsen KE, Rosseland BO, Toften H (2006) Effects of hypo- and hyperoxia on transcription levels of five stress genes and the glutathione system in liver of Atlantic cod (Gadus morhua). J Exp Biol 209:2893–2901
Perry SF, Gilmour KM (2006) Acid-base balance and CO2 excretion in fish: unanswered questions and emerging models. Respir Physiol Neurobiol 154:199–215
Pidetcha P, Ornvichian S, Chalachiva S (2000) Accuracy and precision of the i-STAT portable clinical analyzer: an analytical point of view. J Med Assoc Thai 83(4):445–450
Powell MD, Perry SF (1997) Respiratory and acid-base pathophysiology of hydrogen peroxide in rainbow trout (Oncorhynchus mykiss Walbaum). Aquat Toxicol 37:99–112
Ritola O, Tossavainen K, Kiuru T, Lindström-Seppä P, Mölsä H (2002) Effects of continuous and episodic hyperoxia on stress and hepatic glutathione levels in one-summer-old rainbow trout (Oncorhynchus mykiss). J Appl Ichthyol 18:159–164
Saroglia M, Cecchini S, Terova G, Caputo A, De Stradis A (2000) Influence of environmental temperature and water oxygen concentration on gas diffusion distance in sea bass (Dicentrarchus labrax, L.). Fish Physiol Biochem 23:55–58
Soivio A, Nyholm K, Westman K (1975) A technique for repeated sampling of the blood of individual resting fish. J Exp Biol 63:207–217
Soncini R, Glass ML (2000) Oxygen and acid-base status related drives to gill ventilation in carp. J Fish Biol 56:528–541
Sunde J, Kiessling A, Higgs D, Opstvedt J, Venturini G, Rungruangsak-Torrissen K (2003) Evaluation of feed protein quality by measuring plasma free amino acids in Atlantic salmon (Salmo salar L.) after dorsal aorta cannulation. Aquac Nutr 9:351–360
Takeda T (1990) Ventilation, cardiac output and blood respiratory parameters in the carp, Cyprinus carpio, during hyperoxia. Respir Physiol 81:227–239
Thomas S, Fievet B, Claireaux G, Motais R (1988) Adaptive respiratory responses of trout to acute hypoxia. I—Effects of water ionic composition on blood acid–base status response and gill morphology. Respir Physiol 74:77–90
Wheatly MG, Hòbe H, Wood CM (1984) The mechanisms of acid-base and ionoregulation in the freshwater rainbow trout during environmental hyperoxia and subsequent normoxia, II: the role of the kidney. Respir Physiol 55:155–173
Wood CM, Jackson EB (1980) Blood acid–base regulation during environmental hyperoxia in the rainbow trout (Salmo gairdneri). Respir Physiol 42:351–372
Acknowledgments
The authors would like to thank the staff of NIVA Research station at Solbergstrand for technical assistance. Carlos Salas kindly supplied drawings of the experimental setup. Dr. Carolyn Knight is acknowledged for improving the language of the manuscript. Financial support for the project was provided by the Norwegian Research Council (NRC) grant no. 153202/120.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kristensen, T., Rosseland, B.O., Kiessling, A. et al. Lack of arterial PO2 downregulation in Atlantic salmon (Salmo salar L.) during long-term normoxia and hyperoxia. Fish Physiol Biochem 36, 1087–1095 (2010). https://doi.org/10.1007/s10695-010-9386-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-010-9386-7