Abstract
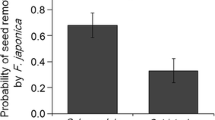
Literature on seed dispersal mutualisms suggests that plant populations should hardly adapt to their current dispersers. We address the predictions that selection pressures exerted by ants on dispersal-related diaspore traits of the ant-dispersed Helleborus foetidus are highly variable in space, and that geographic (inter-population) variation in these traits is unrelated to selection by current dispersers. To test these predictions we use the concept of the quantitative adaptive landscape for seed size at dispersal. Such landscape depicts the relationship between the population’s mean trait value (mean seed size in the present study) and the population’s mean fitness (mean dispersal probability in the present study). Adaptive landscapes make it possible to assess whether the mean population’s phenotype agrees with one favored by selection. We first analyse, in 12 populations of H. foetidus from southern Spain, the extent of divergence among populations in seed and elaiosome size, and the abundance, composition, and behavior of the ant communities. Seeds from a fixed set of five of these populations were offered to ants in all the study sites to fit the adaptive landscape for seed size. In addition, seeds from the local population were also offered in each site. Our results show that seed size has undergone a larger divergence among populations than elaiosome size. Despite geographic variation in ant assemblages, the adaptive landscapes for seed size at dispersal were remarkably similar among sites: ants create disruptive selection on seed size in 10 out of 12 study sites. As predicted, the basic features of these adaptive landscapes (curvature and location of the minimum) varied geographically in accordance with variation in the size of seed dispersers. Also as predicted, in most populations, the observed mean seed size does not agree with that expected from the adaptive landscapes at dispersal. However, the relevance of dispersers for seed size evolution should not be neglected since the agreement between observed and optimum seed size was stronger where dispersers were more abundant. Thus, against the general view, our results evidence that, in H. foetidus, the observed geographic variation in dispersal-related plant traits is partly linked to selection exerted by current dispersers. Geographic variation in ant assemblages determines both the existence of a selection mosaic and the degree of adjustment of populations to the patterns of selection in the mosaic.


Similar content being viewed by others
References
Alcántara JM, Rey PJ (2003) Conflicting selection pressures on seed size: evolutionary ecology of fruit size in a bird-dispersed tree, Olea europaea. J Evol Biol 16:1168–1176
Armbruster WS (1990) Estimating and testing the shapes of adaptive surfaces: the morphology and pollination of Dalechampia blossoms. Am Nat 135:14–31
Arnold SJ, Pfrender ME, Jones AG (2001) The adaptive landscape as a conceptual bridge between micro- and macroevolution. Genetica 112:9–32
Auld TD, Denham AJ (1999) The role of ants and mammals in dispersal and post-dispersal seed predation of the shrubs Grevillea (Proteaceae). Plant Ecol 144:201–213
Beattie AJ, Culver DC (1981) The guild of myrmecochores in the herbaceous flora of West Virginia forests. Ecology 62:107–115
Beattie AJ, Hughes L (2002) Ant–plant interactions. In: Herrera CM, Pellmyr O (eds) Plant–animal interactions: an evolutionary approach. Blackwell Publishing, Oxford, pp 211–235
Boulay R, Coll-Toledano J, Cerda X (2006) Geographic variations in Helleborus foetidus elaiosome lipid composition: implications for dispersal by ants. Chemoecology 16:1–7
Cerdá X, Retana J, Manzaneda A (1998) The role of competition by dominants and temperatura in the foraging of the subordinate species in Mediterranean ants communities. Oecologia 117:404–412
Christian CE (2001) Consequences of a biological invasion reveal the importance of mutualism for plant communities. Nature 413:635–639
Cottingham KL, Lennon JT, Brown BL (2005) Knowing when to draw the line: designing more informative ecological experiments. Front Ecol Environ 3:145–152
Fenner M (2000) Seeds: the ecology of regeneration in plant communities. CABI Publishing, Wallingford
Garrido JL, Rey PJ, Cerdá X, Herrera CM (2002) Geographical variation in diaspore traits of an ant-dispersed plant (Helleborus foetidus): are ant community composition and diaspore traits correlated? J Ecol 90:446–455
Gandon S, Rousset F (1999) Evolution of stepping-stone dispersal rates. Proc R Soc Lond B 266:2507–2513
Giladi I (2006) Choosing benefits or partners: a review of the evidence for the evolution of myrmecochory. Oikos 112:481–492
Godoy JA, Jordano P (2001) Seed dispersal by animals: exact identification of source trees with endocarp DNA microsatellites. Mol Ecol 10:2275–2283
Herrera CM (1995) Plant-vertebrate seed dispersal systems in the Mediterranean: ecological, evolutionary, and historical determinants. Annu Rev Ecol Syst 26:705–727
Herrera CM (1998) Long-term dynamics of Mediterranean frugivorous birds and fleshy fruits: a 12-year study. Ecol Monogr 68:511–538
Herrera CM (2002) Seed dispersal by vertebrates. In: Herrera CM, Pellmyr O (eds) Plant–animal interactions: an evolutionary approach. Blackwell Publishing, Oxford, pp 185–208
Holt RD, McPeek MA (1996) Chaotic population dynamics favors the evolution of dispersal. Am Nat 148:709–718
Howe HF (1984) Constraints in the evolution of mutualisms. Am Nat 123:764–777
Hughes L, Westoby M (1992a) Fate of seeds adapted for dispersal by ants in Australian sclerophyll vegetation. Ecology 73:1285–1299
Hughes L, Westoby M (1992b) Effects of diaspore characteristics on removal of seeds adapted for dispersal by ants. Ecology 73:1300–1312
Jordano P (1987) Patterns of mutualistic interactions in pollination and seed dispersal: connectance, dependence, asymmetries, and coevolution. Am Nat 129:657–677
Jordano P (1995) Angiosperm fleshy fruits and seed dispersers: a comparative analysis of adaptation and constraints in plant–animal interactions. Am Nat 145:163–191
Kalisz S, Hanzawa FM, Tensor SJ, Thiede DA, Voigt S (1999) Ant-mediated seed dispersal alters patterns of relatedness in a population of Trillium grandiflorum. Ecology 80:2620–2634
Kaspari M (1996) Worker size and seed size selection by harvester ants in a Neotropical forest. Oecologia 105:397–404
Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, Gibert P, Beerli P (2001) The strength of phenotypic selection in natural populations. Am Nat 157:245–261
Lande R (1976) Natural selection and random genetic drift in phenotypic evolution. Evolution 30:314–334
Lande R, Arnold SJ (1983) Measurement of selection on correlated characters. Evolution 37:1210–1226
Levin SA, Muller-Landau HC, Nathan R, Chave J (2003) The ecology and evolution of seed dispersal: a theoretical perspective. Annu Rev Ecol Evol Syst 34:575–604
Manzaneda AJ (2005) Ecología y Evolución de la dispersión de semillas en Helleborus foetidus (Ranunculaceae). Variación geográfica en las interacciones planta–animal. PhD thesis, University of Sevilla, Spain
Manzaneda AJ, Fedriani JM, Rey PJ (2005) Adaptive advantages of myrmecochory: the predator-avoidance hypothesis tested over a wide geographic range. Ecography 28:583–592
Mark S, Olesen JM (1996) Importance of elaiosome size to removal of ant-dispersed seeds. Oecologia 107:95–101
McKey D (1975) The ecology of coevolved seed dispersal systems. In: Gilbert LE, Raven PH (eds) Coevolution of animals and plants. University of Texas Press, Austin, pp 159–191
Mitchell-Olds T, Shaw RG (1987) Regression analysis of natural selection: statistical inference and biological interpretation. Evolution 41:1149–1161
Moles AT, Westoby M (2006) Seed size and plant strategy across the whole life cycle. Oikos 113:91–105
Møller AP, Jennions MD (2002) How much variance can be explained by ecologists and evolutionary biologists? Oecologia 132:492–500
Peters M, Oberrath R, Böhning-Gaese K (2003) Seed dispersal by ants: are seed preferences influenced by foraging strategies or historical constraints? Flora 198:413–420
Rey PJ, Ramírez JM, Sánchez-Lafuente AM (2006) Seed- vs. microsite-limited recruitment in a myrmecochorous herb. Plant Ecol 184:213–222
SAS Institute (1998) SAS System for Windows. Version 8.0. Cary, North Carolina, USA
Schluter D (2000) The ecology of adaptive radiation. Oxford University Press, Oxford
Snow DW (1971) Evolutionary aspects of fruit-eating by birds. Ibis 113:194–202
Stanton ML (2003) Interacting guilds: moving beyond the pairwise perspective on mutualisms. Am Nat 162:S10–S23
StatSoft (2001) Statistica for Windows. StatSoft Inc., Tulsa
Steury TD, Murray DL (2005) Regression versus ANOVA. Front Ecol Environ 3, 356–357
Thompson JN (1994) The coevolutionary process. University of Chicago Press, Chicago
Whitlock MC, Phillips PC, Moore FB-G, Tonsor SJ (1995) Multiple fitness peaks and epistasis. Annu Rev Ecol Sys 26:601–629
Willson MF, Traveset A (2000) The ecology of seed dispersal. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities, 2nd edn. CABI Publishing, Wallingford, pp 85–110
Wright S (1943) Isolation by distance. Genetics 28:114–138
Acknowledgments
This study was supported by Ministerio de Ciencia y Tecnología (Spain) Grant BOS2000-1122-C03. R.B. was funded by the European Commission (Marie Curie Individual Fellowship number HPMF-CT-2002-01565). Ant species were determined by Alberto Tinaut. José A. Dorante and Ibama Pineda helped during field work. Jonathan B. Losos and Mark W. Blows provided helpful comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alcántara, J.M., Rey, P.J., Manzaneda, A.J. et al. Geographic variation in the adaptive landscape for seed size at dispersal in the myrmecochorous Helleborus foetidus . Evol Ecol 21, 411–430 (2007). https://doi.org/10.1007/s10682-006-9110-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-006-9110-3