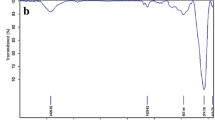
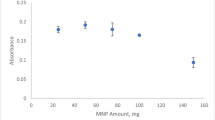
Abstract
The emergence of magnetic materials has opened up doors to numerous applications including their use as sorbents for preconcentration of trace elements. Magnetic materials exhibit many unique advantages in sample preparation such as easy separation from the sample, high preconcentration factor, and short operation period. In the present study, magnetic cobalt material was synthesized, characterized, and used as an effective sorbent in a solid phase extraction process. Experimental variables of the extraction process including pH and volume of buffer solution, eluent concentration and volume, mixing type and period, and sorbent amount were optimized to achieve maximum extraction efficiency. Instrumental variables of flame atomic absorption spectrophotometry and the type of slotted quartz tube were also investigated. Under the optimum conditions, the combined method provided a wide linear range between 50 and 200 ng/mL with detection and quantification limits of 15.4 ng/mL and 51.3 ng/mL, respectively. Relative standard deviations of the proposed method were less than 5.0% and a high enrichment factor of 86.7 was obtained. The proposed method was successfully applied to soil samples for the determination of trace tellurium.




Similar content being viewed by others
References
Afkhami, A., & Norooz-Asl, R. (2009). Removal, preconcentration and determination of Mo (VI) from water and wastewater samples using maghemite nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 346, 52–57. https://doi.org/10.1016/j.colsurfa.2009.05.024.
Amjadi, M., Manzoori, J. L., & Abulhassani, J. (2011). Ultrasound-assisted ionic liquid-based microextraction for preconcentration of arsenic prior to its determination by electrothermal atomic absorption spectrometry. Current Analytical Chemistry, 7, 262–268. https://doi.org/10.2174/1573411011107030262.
Ansari, S., Bhor, R., Pai, K., Sen, D., Mazumder, S., Ghosh, K., Kolekar, Y., & Ramana, C. (2017). Cobalt nanoparticles for biomedical applications: Facile synthesis, physiochemical characterization, cytotoxicity behavior and biocompatibility. Applied Surface Science, 414, 171–187. https://doi.org/10.1016/j.apsusc.2017.03.002.
Ashino, T., & Takada, K. (1995). Determination of trace amounts of selenium and tellurium in nickel-based heat-resisting superalloys, steels and several metals by electrothermal atomic absorption spectrometry after reductive coprecipitation with palladium using ascorbic acid. Analytica Chimica Acta, 312, 157–163. https://doi.org/10.1016/0003-2670(95)00215-L.
Ba, L. A., Döring, M., Jamier, V., & Jacob, C. (2010). Tellurium: an element with great biological potency and potential. Organic & Biomolecular Chemistry, 8, 4203–4216. https://doi.org/10.1039/c0Ob00086h.
Cerwenka, E. A., Jr., & Cooper, W. C. (1961). Toxicology of selenium and tellurium and their compounds. Archives of Environmental Health: An International Journal, 3, 189–200. https://doi.org/10.1080/00039896.1961.10663003.
Cullity, B. D. (1978). Elements of X-ray diffraction. https://doi.org/10.1021/ed034pA178.
El-Shahawi, M., Al-Saidi, H., Al-Harbi, E., Bashammakh, A., & Alsibbai, A. (2013). Speciation and determination of tellurium in water, soil, sediment and other environmental samples. In S. Bakirdere (Ed.), Speciation Studies in Soil, Sediment and Environmental Samples (pp. 527–544). https://doi.org/10.1201/b15501-15.
Ghasemi, E., Najafi, N. M., Raofie, F., & Ghassempour, A. (2010). Simultaneous speciation and preconcentration of ultra traces of inorganic tellurium and selenium in environmental samples by hollow fiber liquid phase microextraction prior to electrothermal atomic absorption spectroscopy determination. Journal of Hazardous Materials, 181, 491–496. https://doi.org/10.1016/j.jhazmat.2010.05.040.
Giakisikli, G., & Anthemidis, A. N. (2013). Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Analytica Chimica Acta, 789, 1–16. https://doi.org/10.1016/j.aca.2013.04.021.
Grotti, M., Lagomarsino, C., & Frache, R. (2005). Multivariate study in chemical vapor generation for simultaneous determination of arsenic, antimony, bismuth, germanium, tin, selenium, tellurium and mercury by inductively coupled plasma optical emission spectrometry. Journal of Analytical Atomic Spectrometry, 20, 1365–1373. https://doi.org/10.1039/B510803A.
Guo, F., Zheng, H., Yang, Z., & Qian, Y. (2002). Synthesis of cobalt nanoparticles in ethanol hydrazine alkaline system (EHAS) at room temperature. Materials Letters, 56, 906–909. https://doi.org/10.1016/S0167-577X(02)00635-3.
Harada, T., & Takahashi, Y. (2008). Origin of the difference in the distribution behavior of tellurium and selenium in a soil–water system. Geochimica et Cosmochimica Acta, 72, 1281–1294. https://doi.org/10.1016/j.gca.2007.12.008.
Hritcu, D., Dodi, G., & Popa, M. I. (2012). Heavy metal ions adsorption on chitosan-magnetite microspheres. International Review of Chemical Engineering, 4, 364–368 Special Section on Open Door Initiative (ODI)-2nd edition.
Huang, C., & Hu, B. (2008). Speciation of inorganic tellurium from seawater by ICP-MS following magnetic SPE separation and preconcentration. Journal of Separation Science, 31, 760–767. https://doi.org/10.1002/jssc.200700405.
Kaplan, M., Cerutti, S., Moyano, S., Olsina, R., Martinez, L., & Gásquez, J. (2004). On-line preconcentration system by coprecipitation with lanthanum hydroxide using packed-bed filter for the determination of tellurium in water by ICP-OES with USN. Instrumentation Science & Technology, 32, 423–431. https://doi.org/10.1081/ci-120037674.
Körez, A., Eroğlu, A. E., Volkan, M., & Ataman, O. Y. (2000). Speciation and preconcentration of inorganic tellurium from waters using a mercaptosilica microcolumn and determination by hydride generation atomic absorption spectrometry. Journal of Analytical Atomic Spectrometry, 15, 1599–1605. https://doi.org/10.1039/b004696p.
Leiber, M. A. (2005). Use of tellurium in carbon-supported, noble metal-containing catalysts for liquid phase oxidation reactions. Google Patents. WO/2004/002622.
Lin, Z. H., Yang, Z., & Chang, H. T. (2007). Preparation of fluorescent tellurium nanowires at room temperature. Crystal Growth and Design, 8, 351–357. https://doi.org/10.1021/cg070357f.
Lu, Q., Gao, F., & Komarneni, S. (2005). A green chemical approach to the synthesis of tellurium nanowires. Langmuir, 21, 6002–6005. https://doi.org/10.1021/la050594p.
Ma, Y., Hao, Q., Poudel, B., Lan, Y., Yu, B., Wang, D., Chen, G., & Ren, Z. (2008). Enhanced thermoelectric figure-of-merit in p-type nanostructured bismuth antimony tellurium alloys made from elemental chunks. Nano Letters, 8, 2580–2584. https://doi.org/10.1021/nl8009928.
Nyaba, L., Biata, N. R., Ngila, J. C., & Nomngongo, P. N. (2017). Ultrasound assisted-ionic liquid-dispersive liquid-liquid microextraction for preconcentration of inorganic tellurium in environmental water samples prior to inductively coupled plasma–optical emission spectrometry detection. Journal of Molecular Liquids, 231, 154–159. https://doi.org/10.1016/j.molliq.2017.02.012.
Pedro, J., Stripekis, J., Bonivardi, A., & Tudino, M. (2008). Determination of tellurium at ultra-trace levels in drinking water by on-line solid phase extraction coupled to graphite furnace atomic absorption spectrometer. Spectrochimica Acta Part B: Atomic Spectroscopy, 63, 86–91. https://doi.org/10.1016/j.sab.2007.11.011.
Šafařı́ková, M., & Šafařı́k, I. (1999). Magnetic solid-phase extraction. Journal of Magnetism and Magnetic Materials, 194, 108–112. https://doi.org/10.1016/S0304-8853(98)00566-6.
Schramel, P., Wendler, I., & Angerer, J. (1997). The determination of metals (antimony, bismuth, lead, cadmium, mercury, palladium, platinum, tellurium, thallium, tin and tungsten) in urine samples by inductively coupled plasma-mass spectrometry. International Archives of Occupational and Environmental Health, 69, 219–223.
Takenaga, M., Yamada, N., Ohara, S., Nishiuchi, K., Nagashima, M., Kashihara, T., Nakamura, S., & Yamashita, T. (1983). New optical erasable medium using tellurium suboxide thin film (pp. 173–178). Bellingham: Optical Storage Media. International Society for Optics and Photonics. https://doi.org/10.1117/12.936065.
Urbánková, K., Moos, M., Machát, J., & Sommer, L. (2011). Simultaneous determination of inorganic arsenic, antimony, selenium and tellurium by ICP-MS in environmental waters using SPE preconcentration on modified silica. International Journal of Environmental Analytical Chemistry, 91, 1077–1087. https://doi.org/10.1080/03067311003782666.
Xu, P., Li, J., Shi, L., Selke, M., Chen, B., & Wang, X. (2013). Synergetic effect of functional cadmium–tellurium quantum dots conjugated with gambogic acid for HepG2 cell-labeling and proliferation inhibition. International Journal of Nanomedicine, 8, 3729. https://doi.org/10.2147/IJN.S51622.
Yang, G., Zheng, J., Tagami, K., & Uchida, S. (2013). Rapid and sensitive determination of tellurium in soil and plant samples by sector-field inductively coupled plasma mass spectrometry. Talanta, 116, 181–187. https://doi.org/10.1016/j.talanta.2013.05.015.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Özdoğan, N., Kapukıran, F., Öztürk Er, E. et al. Magnetic cobalt particle–assisted solid phase extraction of tellurium prior to its determination by slotted quartz tube-flame atomic absorption spectrophotometry. Environ Monit Assess 191, 339 (2019). https://doi.org/10.1007/s10661-019-7490-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7490-4