Abstract
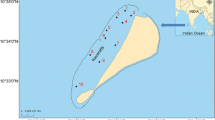
The study addressed the impact of the El Niño 2015–2016 on the ecosystem functioning and the subsequent effects on the distribution and community structure of zooplankton in the Kavaratti reef, a prominent coral atoll in the tropical Indian Ocean. The elevated ocean temperature (SST) associated with El Niño resulted in a mass bleaching event affecting > 60% of the live corals of the Kavaratti atoll. The concomitant changes observed in the nutrient concentration, coral health, and phytoplankton of the reef environment during the course of the El Niño led to discernible variations in the zooplankton community with markedly higher abundance and heterogeneity in distribution during the peak period of El Niño compared to its waning phase. A notable shift was also evident in the community structure of Copepoda, the dominant zooplankton taxon, with a predominance of calanoids and poecilostomatoids in the peak period and by harpacticoid copepods in the waning phase of the El Niño. The harpacticoid, Macrosetella gracilis, dominated in the waning phase because of their unique adaptability in the utilization of Trichodesmium erythraeum, both as nutritional and physical substrates in the nutrient-depleted environment of the reef ecosystem.










Similar content being viewed by others
References
Achuthankutty, C. T., Nair, S. R., Haridas, P., & Madhupratap, M. (1989). Zooplankton composition of the Kalpeni and Agatti atolls, Lakshadweep archipelago. Indian Journal of Marine Science, 18, 151–154.
Armbrust, E. V. (2009). The life of diatoms in the world’s oceans. Nature, 459, 185–192.
Arthur, R. (2000). Coral bleaching and mortality in three Indian reef regions during an El Niño southern oscillation event. Current Science, 79, 1723–1729.
Baker, A. C., Glynn, P. W., & Riegl, B. (2008). Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuarine Coastal and Shelf Science, 80, 435–471.
Bakun, A., & Broad, K. (2003). Environmental ‘loopholes’ and fish population dynamics: comparative pattern recognition with focus on El Nino effects in the Pacific. Fisheries Oceanography, 12, 458–473.
Barton, B. A., Morgan, J. D., Vijayan, M. M., & Adams, S. M. (2002). Physiological and condition-related indicators of environmental stress in fish. In S. M. Adams (Ed.), Biological indicators of aquatic ecosystem stress (pp. 111–148). Bethesda: American Fisheries Society.
Bergman, B., Sandh, G., Lin, S., Larsson, J., & Carpenter, E. J. (2013). Trichodesmium—a widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS Microbiology Reviews, 37, 286–302.
Bjӧrnberg, T. K. S. (1965). Observations on the development and the biology of the Miracidae Dana (Copepoda: Crustacea). Bulletin of Marine Science, 15, 512–520.
Bӧttger-Schnack, R., & Schnack, D. (1989). Vertical distribution and population structure of Macrosetella gracilis (Copepoda: Harpacticoida) in the Red Sea in relation to the occurrence of Oscillatoria (Trichodesmium) spp (Cyanobacteria). Marine Ecology Progress Series, 52, 17–31.
Bravo, I., Fernandez, M. L., & Martinez, R. A. (2001). Toxin composition of the toxic dinoflagellate Prorocentrum lima isolated from different locations along the Galician coast (NW Spain). Toxicon, 39, 1537–1545.
Brown, B. E., & Bythell, J. C. (2005). Perspectives on mucus secretion in reef corals. Marine Ecology Progress Series, 296, 291–309.
Carpenter, E. J., & Price, C. C. (1977). Nitrogen fixation, distribution, and production of Oscillatoria (Trichodesmium) spp. in the western Sargasso and Caribbean Seas. Limnology and Oceanography, 22, 60–72.
Chen, S., Wu, R., Chen, W., Yu, B., & Cao, X. (2016). Genesis of westerly wind bursts over the equatorial western Pacific during the onset of the strong 2015–2016 El Niño. Atmospheric Science Letters, 17, 384–391.
Claar, D. C., Szostek, L., McDevitt-Irwin, J. M., Schanze, J. J., & Baum, J. K. (2018). Global patterns and impacts of El Niño events on coral reefs: a meta-analysis. PLoS One, 13(2), e0190957.
Clarke, K. R., & Gorley, R. N. (2015). PRIMER v7: user manual/tutorial. Plymouth: PRIMER-E.
Clifford, H. T., & Stephensen, W. (1975). An introduction to numerical classification. New York: Academic Press.
Coles, S. L., & Strathmann, R. (1973). Observations on coral mucus “flocs” and their potential trophic significance. Limnology and Oceanography, 18, 673–678.
Conway, D. V. P., White, R. G., Hugues-Dit-Ciles, J., Gallienne, C. P., & Robins, D. B. (2003). Guide to the coastal and surface zooplankton of the South-Western Indian Ocean. Plymouth: Marine Biological Association of the United Kingdom.
Costello, M. J., & Chaudhary, C. (2017). Marine biodiversity, biogeography, deep-sea gradients, and conservation. Current Biology, 27, R511–R527.
De Goeij, J. M., Van Oevelen, D., Vermeij, M. J., Osinga, R., Middelburg, J. J., de Goeij, A. F., & Admiraal, W. (2013). Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science, 342, 108–110.
Diaz-Pulido, G., McCook, L. J., Dove, S., Berkelmans, R., Roff, G., Kline, D. I., Weeks, S., Evans, R. D., Williamson, D. H., & Hoegh-Guldberg, O. (2009). Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS One, 4(4), e5239.
Ducklow, H. W., & Mitchell, R. (1979). Composition of mucus released by coral reef coelenterates. Limnology and Oceanography, 24, 706–714.
Eakin, M. (2016). Outlook on coral bleaching: El Niño, the guest overstaying his welcome. Presentation in 35 Meeting Washington DC, February, 2016. https://www.coralreef.gov/meeting35/pdf/7_2016_Outlook_on_Coral_Bleaching.
Eberl, R., Cohen, S., Cipriano, F., & Carpenter, E. J. (2007). Genetic diversity of the pelagic harpacticoid copepod Macrosetella gracilis on colonies of the cyanobacterium Trichodesmium spp. Aquatic Biology, 1, 33–43.
Edwards, M., & Richardson, A. J. (2004). Impact of climate change on marine pelagic phenology and trophic mismatch. Nature, 430, 881–884.
Gerber, R., & Gerber, M. (1979). Ingestion of natural particulate organic matter and subsequent assimilation, respiration and growth by tropical lagoon zooplankton. Marine Biology, 52, 33–43.
Glynn, P. W. (1988). El Niño-Southern Oscillation 1982-1983: nearshore population, community, and ecosystem responses. Annual Review of Ecology Evolution and Systematics, 19, 309–346.
Glynn, P. W., Mones, A. B., Podestá, G. P., Colbert, A., & Colgan, M. W. (2017). El Niño-Southern Oscillation: Effects on Eastern Pacific coral reefs and associated biota. In P. W. Glynn, D. P. Manzello, & I. C. Enochs (Eds.), Coral reefs of the Eastern Tropical Pacific (pp. 251–290). Netherlands: Springer.
Gottfried, M., & Roman, M. R. (1983). Ingestion and incorporation of coral-mucus detritus by reef zooplankton. Marine Biology, 72, 211–218.
Grasshoff, K. (1983). Determination of oxygen. In K. Grasshoff, M. Ehrhardt, & K. Kremling (Eds.), Methods of sea water analysis (pp. 61–72). Weinheim: Verlag Chemie.
Hagen, W. (2000). Biovolume and biomass determinations. In R. Harris, P. Wiebe, J. Lenz, H. R. Skjoldal, & M. E. Huntley (Eds.), ICES zooplankton methodology manual (pp. 87–147). London: Academic Press.
Hancock, G. J., Webster, I., & Stieglitz, T. C. (2006). Horizontal mixing of Great Barrier Reef waters: offshore diffusivity determined from radium isotope distribution. Journal of Geophysical Research Oceans, 111, C12019. https://doi.org/10.1029/2006JC003608.
Haridas, P., & Madhupratap, M. (1977). Acartia dweepi, a new species of copepod (Acartidae, galanoida) from Lakshadweep. Current Science, 47, 176–177.
Harris, R., Wiebe, P., Lenz, J., Skjoldal, H. R., & Huntley, M. E. (Eds.). (2000). ICES zooplankton methodology manual. London: Academic Press.
Hays, G. C., Richardson, A. J., & Robinson, C. (2005). Climate change and marine plankton. Trends in Ecology and Evolution, 20, 337–344.
Hecky, R. E., & Kilham, P. (1988). Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnology and Oceanography, 33, 796–822.
Heidelberg, K. B., O'Neil, K. L., Bythell, J. C., & Sebens, K. P. (2010). Vertical distribution and diel patterns of zooplankton abundance and biomass at Conch Reef, Florida Keys (USA). Journal of Plankton Research, 32, 75–91.
Houlbrèque, F., Tambutté, E., Allemand, D., & Ferrier-Pagès, C. (2004). Interactions between zooplankton feeding, photosynthesis and skeletal growth in the scleractinian coral Stylophora pistillata. Journal of Experimental Biology, 207, 1461–1469.
James, P. S. B. R. (2011). The Lakshadweep: islands of ecological fragility, environmental sensitivity and anthropogenic vulnerability. Journal of Coastal Environment, 2, 9–25.
Kämpf, J., & Chapman, P. (2016). Upwelling systems of the world. A scientific journey to the most productive marine ecosystem. Switzerland: Springer.
Karati, K. K., Vineetha, G., Madhu, N. V., Anil, P., Dayana, M., Shihab, B. K., Muhsin, A. I., Riyas, C., & Raveendran, T. V. (2017). Variability in the phytoplankton community of Kavaratti reef ecosystem (northern Indian Ocean) during peak and waning periods of El Niño 2016. Environmental Monitoring and Assessment, 189, 1–17.
Kelley, R. (2009). Indo Pacific coral finder. See www.byoguides.com.
Lefcheck, J. S., Byrnes, J. E., Isbell, F., et al. (2015). Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nature Communications, 6, 1–7.
Luo, J. J., Zhang, R., Behera, S. K., Masumoto, Y., Jin, F. F., Lukas, R., & Yamagata, T. (2010). Interaction between El Nino and extreme Indian ocean dipole. Journal of Climate, 23, 726–742.
Madhupratap, M., Achuthankutty, C. T., & Nair, S. S. (1991). Zooplankton of the lagoons of the Laccadives: diel patterns and emergence. Journal of Plankton Research, 13, 947–958.
McPhaden, M. J. (2004). Evolution of the 2002/03 El Niño. Bulletin of the American Meteorological Society, 85, 677–695.
McPhaden, M. J., Zebiak, S. E., & Glantz, M. H. (2006). ENSO as an integrating concept in earth science. Science, 314, 1740–1745.
Morton, S. L., & Tindall, D. R. (1995). Morphological and biochemical variability of the toxic dinoflagellate Prorocentrum lima isolated from three locations at Heron Island, Australia. Journal of Phycology, 31, 914–921.
Muraleedharan, P. M., & Prasannakumar, S. (1996). Arabian Sea upwelling—a comparison between coastal and open ocean regions. Current Science, 71, 842–846.
O'Neil, J. M. (1998). The colonial cyanobacterium Trichodesmium as a physical and nutritional substrate for the harpacticoid copepod Macrosetella gracilis. Journal of Plankton Research, 20, 43–59.
O'Neil, J. M., & Roman, M. R. (1994). Ingestion of the cyanobacterium Trichodesmium spp. by pelagic harpacticoid copepods Macrosetella, Miracia and Oculosetella. Hydrobiologia, 292, 235–240.
Richardson, A. J. (2008). In hot water: zooplankton and climate change. ICES Journal of Marine Science, 65, 279–295.
Richman, S., Loya, Y., & Sloboclkin, L. B. (1975). The rate of mucus production by corals and its assimilation by the coral reef copepod Acartia negligens. Limnology and Oceanography, 20, 918–923.
Seckbach, J., & Kociolek, J. P. (Eds.). (2011). The diatom world. New York: Springer.
SenGupta, R., Mores, C., Kureishy, T. W., Sankaranarayanan, V. N., Jana, T. K., Naqvi, S. W. A., & Rajagopal, M. D. (1979). Chemical oceanography of the Arabian Sea: part IV—Laccadive Sea. Indian Journal of Geo-Marine Sciences, 8, 215–221.
Sewell, R. B. S. (1999). The copepod of Indian seas. India: Daya Books.
Shenoi, S. S. C., Shankar, D., & Shetye, S. R. (1999). On the sea surface temperature high in the Lakshadweep Sea before the onset of the southwest monsoon. Journal of Geophysical Research Oceans, 104(C7), 15703–15712.
Sohm, J. A., & Capone, D. G. (2006). Phosphorus dynamics of the tropical and subtropical North Atlantic: Trichodesmium spp. versus bulk plankton. Marine Ecology Progress Series, 317, 21–28.
Sommer, U. (2000). Scarcity of medium-sized phytoplankton in the northern Red Sea explained by strong bottom-up and weak top-down control. Marine Ecology Progress Series, 197, 19–25.
Stramma, L., Fischer, T., Grundle, D. S., Krahmann, G., Bange, H. W., & Marandino, C. A. (2016). Observed El Niño conditions in the eastern tropical Pacific in October 2015. Ocean Science, 12, 861–873.
Tomas, C. R. (1997). Identifying marine phytoplankton. New York: Academic Press.
Trenberth, K. E., Branstator, G. W., Karoly, D., Kumar, A., Lau, N. C., & Ropelewski, C. (1998). Progress during TOGA in understanding and modeling global teleconnections associated with tropical sea surface temperatures. Journal of Geophysical Research Oceans, 103(C7), 14291–14324.
Van den Wollenberg, A. L. (1977). Redundancy analysis. An alternative for canonical correlation analysis. Psychometrika, 42, 207–219.
Vijay Anand, P. E., & Pillai, N. G. K. (2007). Coral reef fish abundance and diversity of seagrass beds in Kavaratti atoll, Lakshadweep, India. Indian Journal of Fisheries, 54, 11–20.
Walther, G. R., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T. J., Fromentin, J. M., Hoegh-Guldberg, O., & Bairlein, F. (2002). Ecological responses to recent climate change. Nature, 416, 389–395.
Warwick, R. M., Clarke, K. R., & Somerfield, P. J. (2008). k-Dominance curves. In S. E. Jørgensen & B. D. Fath (Eds.), Ecological indicators. 3, encyclopedia of ecology, 5 (pp. 2055–2057). Oxford: Elsevier.
Wilson, S. G., Taylor, J. G., & Pearce, A. F. (2001). The seasonal aggregation of whale sharks at Ningaloo Reef, Western Australia: currents, migrations and the El Nino/Southern Oscillation. Environmental Biology of Fishes, 61, 1–11.
Acknowledgements
Our sincere thanks to CSIR-NIO, CMFRI, and DST, Lakshadweep for the facilities provided. KKK is thankful to CSIR for a post-doctoral fellowship. This is NIO contribution 6260.
Funding
This research program was supported by the Institutional project OLP 1210 of CSIR-NIO.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Figure 1
The interrelation of the zooplankton abundance with the various size structured phytoplankton biomass. (PNG 11 kb)
High Resolution Image
(TIF 34 kb)
Rights and permissions
About this article
Cite this article
Vineetha, G., Karati, K.K., Raveendran, T.V. et al. Responses of the zooplankton community to peak and waning periods of El Niño 2015–2016 in Kavaratti reef ecosystem, northern Indian Ocean. Environ Monit Assess 190, 465 (2018). https://doi.org/10.1007/s10661-018-6842-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6842-9