Abstract
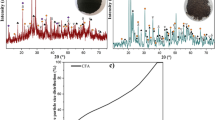
AOD (argon oxygen decarburization) slag is the by-product in the stainless steel refining process. Chromium existing in AOD slag can leach out and probably poses a serious threat to the environment. To assess the leaching toxicity of chromium released from AOD slag, the temperature-dependent maximum availability leaching test was performed. To determine the controlling mineralogical phases of chromium released from AOD slag, a Visual MINTEQ simulation was established based on Vminteq30 and the FactSage 7.0 database. The leaching tests indicated that the leaching availability of chromium was slight and mainly consisted of trivalent chromium. Aging of AOD slag under the atmosphere can oxidize trivalent chromium to hexavalent chromium, which could be leached out by rainwater. According to the simulation, the chromium concentration in leachates was controlled by the freely soluble pseudo-binary phases in the pH = 7.0 leaching process and controlled by the Cr2O3 phase in the pH = 4.0 leaching process. Chromium concentrations were underestimated when the controlling phases were determined to be FeCr2O4 and MgCr2O4. Facilitating the generation of the insoluble spinel-like phases during the cooling and disposal process of the molten slag could be an effective approach to decreasing the leaching concentration of chromium and its environmental risk.




Similar content being viewed by others
References
Adegoloye, G., Beaucour, A. L., Ortola, S., & Noumowe, A. (2015). Concretes made of EAF slag and AOD slag aggregates from stainless steel process: mechanical properties and durability. Construction and Building Materials, 76, 313–321.
Amita, D. A., Vinod, T., & Purnendu, B. (2006). Extent of oxidation of Cr(III) to Cr(VI) under various conditions pertaining to natural environment. Journal of Hazardous Materials, 128, 164–174.
Bartlett, R. J. (1991). Chromium cycling in soils and water: links, gaps and methods. Environmental Health Perspectives, 92, 31–34.
Beverskog, B., & Puigdomenech, I. (1997). Revised pourbaix diagrams for chromium at 25–300 °C. Corros. Sci., 29, 43–57.
Bissen, M., & Frimmel, F. H. (2003). Arsenic—a review. Part 2: oxidation of arsenic and its removal in water treatment. Acta Hydrochimica et Hydrobiologica, 31, 97–107.
Chen, Y. W., & Tian, S. (2008). Improvement of determing total chrome with spectrophotom etricmethod. J. HLJ. Environ., 32, 61–66.
Dhal, B., Abhilash, & Pandey, B. D. (2013). Process optimization for bio-beneficiation of a chromite concentrate by a Cr(VI) reducing native microbe (Bacillus sp.). International Journal of Mineral Processing, 123, 129–136.
EA NEN 7371, 2004. Leaching characteristicsof granular building and waste materials. The determination of the availability of inorganic components for leaching. The maximum availability leaching test. Based on a translation of the Netherlands normalisation institute standard.
Fang, J. Z., & Xu, C. F. (2010). Study on three kinds of XRD quantitative analysis methods. Coal Conversion., 33, 88–91.
Halim, C. E., Short, S. A., Scott, J. A., et al. (2005). Modelling the leaching of Pb, Cd, As, and Cr from cementitious waste using PHREEQC. Journal of Hazardous Materials, 125, 45–61.
Hui, W., Baijun, Y., & Fan, L. (2015). Analysis of Cr with various valence states in industrial EAF slag for making stainless steel. ISIJ International, 55, 1425–1431.
Jing, C. Y., Liu, S. Q., George, P. K., & Meng, X. G. (2006). Leaching behavior of Cr(III) in stabilized/solidified soil. Chemosphere, 64, 379–385.
Kozo, S., Hirotaka, H., Nobuhiro, M., et al. (2008). Chemical state of chromium in CaO–SiO2 base oxides annealed under different conditions. ISIJ International, 48, 1404–1408.
Kuhn, M., Mudersbach, D., 2004. Treatment of liquid EAF-slag from stainless steelmaking to produce environment friendly construction materials. Processings of the 2nd international conference on process development in iron and steelmaking.
Loncnar, M., Zupan, M., Bukovec, P., & Jakli, A. (2009). The effect of water cooling on the leaching behaviour of EAF slag from stainless steel production. Materials and Technologies, 43, 315–321.
Lee, T., Lim, H., Lee, Y., & Park, J. (2003). Use of waste iron for removal of Cr(VI) from water. Chemosphere, 53, 479–485.
Li, J.L., Li, M.X., Wu, P.F., et al., 2015. Effect of slag components on chromium occurrence in stainless steel slag. Processings of the 10th conference of Baosteel. 1–5.
Liu, B., Li, J. G., Zeng, Y. N., & Wang, Z. M. (2016). Toxicity assessment and geochemical model of chromium leaching from AOD slag. Chemosphere, 144, 2052–2057.
Liu, F. L., & Liu, X. R. (2009). Removal of chromium (VI) with zero valent iron: an experimental research. Journal of Environmental Health, 26, 139–141.
Leuz, A. K., & Johnson, C. A. (2005). Oxidation of SbIII to SbV by O2 and H2O2 in aqueous solutions. Geochimica et Cosmochimica Acta, 69, 1165–1172.
Nie, N., Ding, Y. Z., & Li, X. Q. (2013). Preparation of zeolite and zero valent iron composite for clean up of hexavalent contamination in water. Chin. Environ. Sci., 33, 443–447.
Pei, T. Q., Wang, J. W., & Tsai, C. H. (2008). Pollution characteristics and treatment analysis of chromium residue and soil chromium in typical chromium residue simple stock. Chin. J. Environ. Eng., 2, 994–999.
Samada, Y., Miki, T., & Hino, M. (2011). Prevention of chromium elution from stainless steel slag into seawater. ISIJ International, 51, 728–732.
Santos, R., Bouwel, J., Vandevelde, E., et al. (2013). Accelerated mineral carbonation of stainless steel slags for CO2 storage and waste valorization: effect of process parameters on geochemical properties. International Journal of Greenhouse Gas Control., 17, 32–45.
Sheen, Y., Wang, H., & Sun, T. (2013). A study of engineering properties of cement mortar with stainless steel oxidizing slag and reducing resource materials. Construction and Building Materials, 40, 239–245.
Sheen, Y., Wang, H., & Sun, T. (2014b). Properties of green concrete containing stainless steel oxidizing slag resource materials. Construction and Building Materials, 50, 22–27.
Sheen, Y., Huang, L., Wang, H., et al. (2014a). Experimental study and strength formulation of soil-based controlled low-strength material containing stainless steel reducing slag. Construction and Building Materials, 54, 1–9.
Wang, R. Y., Chen, R. H., & Shi, L. (2009). Beneficial use of stainless steel EAF slag as composite cement admixture and its heavy metals leaching risk analysis. Bs. Technol. Res., 4, 14–18.
Zhang, H., & Hong, X. (2011). An overview for the utilization of wastes from stainless steel industries. Resources, Conservation and Recycling, 55, 745–754.
Zhang, Y., & Chen, X. Y. (2012). Research on determination of chromium (VI) in surface water environment. Appl. Chem. Ind., 41, 349–351.
Zhen, C. L., Zhao, G., Na, X. Z., et al. (2012). Pollution control of AOD slag. J. Environ. Pollut. control., 34, 76–79.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51274085, 51574108) and the State Key Laboratory of Solid Waste Reuse for Building Materials (SWR-2013-010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Liu, B., Zeng, Y. et al. Maximum availability and mineralogical control of chromium released from AOD slag. Environ Monit Assess 189, 113 (2017). https://doi.org/10.1007/s10661-017-5843-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-5843-4