Abstract
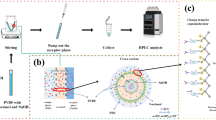
A novel and environmentally friendly ionic-liquid-based hollow-fiber liquid-phase microextraction method combined with a hybrid artificial neural network (ANN)–genetic algorithm (GA) strategy was developed for ferro and ferric ions speciation as model analytes. Different parameters such as type and volume of extraction solvent, amounts of chelating agent, volume and pH of sample, ionic strength, stirring rate, and extraction time were investigated. Much more effective parameters were firstly examined based on one-variable-at-a-time design, and obtained results were used to construct an independent model for each parameter. The models were then applied to achieve the best and minimum numbers of candidate points as inputs for the ANN process. The maximum extraction efficiencies were achieved after 9 min using 22.0 μL of 1-hexyl-3-methylimidazolium hexafluorophosphate ([C6MIM][PF6]) as the acceptor phase and 10 mL of sample at pH = 7.0 containing 64.0 μg L−1 of benzohydroxamic acid (BHA) as the complexing agent, after the GA process. Once optimized, analytical performance of the method was studied in terms of linearity (1.3–316 μg L−1, R 2 = 0.999), accuracy (recovery = 90.1–92.3 %), and precision (relative standard deviation (RSD) <3.1). Finally, the method was successfully applied to speciate the iron species in the environmental and wastewater samples.




Similar content being viewed by others
References
Anthemidis, A. N., Zachariadis, G. A., & Stratis, J. A. (2003). Development of an on-line solvent extraction system for electrothermal atomic absorption spectrometry utilizing a new gravitational phase separator determination of cadmium in natural waters and urine samples. Journal of Analytical at Spectrometry, 18, 1400–1403.
Barfi, B., Asghari, A., Rajabi, M., Sabzalian, S., Khanalipoor, F., & Behzad, M. (2015a). Optimized syringe-assisted dispersive micro solid phase extraction coupled with microsampling flame atomic absorption spectrometry for the simple and fast determination of potentially toxic metals in fruit juice and bio-fluid samples. RSC Advances, 5, 31930–31941.
Barfi, B., Asghari, A., Rajabi, M., Goochani Moghadam, A., Mirkhani, N., & Ahmadi, F. (2015b). Comparison of ultrasound-enhanced air-assisted liquid–liquid microextraction and low-density solvent-based dispersive liquid–liquid microextraction methods for determination of nonsteroidal anti-inflammatory drugs in human urine samples. Journal of Pharmaceutical and Biomedical Analysis, 111, 297–305.
Barfi, B., Asghari, A., Rajabi, M., & Sabzalian, S. (2015c). Organic solvent-free air-assisted liquid–liquid microextraction for optimized extraction of illegal azo-based dyes and their main metabolite from spices, cosmetics and human bio-fluid samples in one step. Journal of Chromatography B, 999, 15–25.
Bautista-Flores, A. N., de San Miguel, E. R., de Gyves, J., & Jönsson, J. Å. (2010). Optimization, evaluation, and characterization of a hollow fiber supported liquid membrane for sampling and speciation of lead (II) from aqueous solutions. Journal of Membrane Science, 363, 180–187.
Bousejera-ElGarah, F., Bijani, C., Coppel, Y., Faller, P., & Hureau, C. (2011). Iron(II) binding to amyloid-β, the Alzheimer’s peptide. Inorganic Chemistry, 50, 9024–9030.
Bridle, K. R., Frazer, D. M., & Wilkins, S. J. (2003). Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet, 361, 669–673.
Chen, C., & Ramaswamy, H. (2002). Modeling and optimization of variable retort temperature (VRT) thermal processing using coupled neural networks and genetic algorithms. Journal of Food Engineering, 53, 209–220.
Citak, D., & Tuzen, M. (2010). A novel preconcentration procedure using cloud point extraction for determination of lead, cobalt and copper in water and food samples using flame atomic absorption spectrometry. Food and Chemical Toxicology, 48, 1399–1404.
Cook, D., Ragsdale, C., & Major, R. (2000). Combining a neural network with a genetic algorithm for process parameter optimization. Engineering Applications of Artificial Intelligence, 13, 391–396.
Crichton, R. R., Dexter, D., & Ward, R. J. (2008). Metal based neurodegenerative diseases—from molecular mechanisms to therapeutic strategies. Coordination Chemistry Reviews, 252, 1189–1199.
Garcia-Marco, S., Torreblanca, A., & Lucena, J. (2006). Chromatographic determination of Fe chelated by ethylenediamine-N-(o-hydroxyphenylacetic)-N'-(p-hydroxyphenylacetic) acid in commercial EDDHA/Fe3+ fertilizers. Journal of Agricultural and Food Chemistry, 54, 1380–1386.
Ghaedi, M., Mortazavi, K., Montazerozohori, M., Shokrollahi, A., & Soylak, M. (2013a). Flame atomic absorption spectrometric (FAAS) determination of copper, iron and zinc in food samples after solid-phase extraction on Schiff base-modified duolite XAD 761. Materials Science and Engineering C, 33, 2338–2344.
Ghaedi, M., Niknam, K., Zamani, S., Abasi-Larki, H., Roosta, M., & Soylak, M. (2013b). Silica chemically bonded N-propyl kriptofix 21 and 22 with immobilized palladium nanoparticles for solid phase extraction and preconcentration of some metal ions. Materials Science and Engineering C, 33, 3180–3189.
Gharahbagh, A. A., & Abolghasemi, V. (2008). A novel accurate genetic algorithm for multivariable systems. World Applied Sciences Journal, 5, 137–142.
Ghasemi, E., Najafi, N. M., Raofie, F., & Ghassempour, A. (2010). Simultaneous speciation and preconcentration of ultra traces of inorganic tellurium and selenium in environmental samples by hollow fiber liquid phase microextraction prior to electrothermal atomic absorption spectroscopy determination. Journal of Hazardous Materials, 181, 491–496.
Giokas, D. L., Paleologos, E. K., & Karayannis, M. I. (2002). Speciation of Fe (II) and Fe (III) by the modified ferrozine method, FIA–spectrophotometry, and flame AAS after cloud-point extraction. Analytical and Bioanalytical Chemistry, 373, 237–243.
Grotti, M., Abelmoschi, M. L., Soggia, F., & Frache, R. (2003). Determination of ultratrace elements in natural waters by solid-phase extraction and atomic spectrometry methods. Analytical and Bioanalytical Chemistry, 375, 242–247.
Guo, X., He, M., Chen, B., & Hu, B. (2012). Phase transfer hollow fiber liquid phase microextraction combined with electrothermal vaporization inductively coupled plasma mass spectrometry for the determination of trace heavy metals in environmental and biological samples. Talanta, 101, 516–523.
Hauser, C. R., & Renfrow, W. B. (1943). Benzohydroxamic acid. Organic Synthesis, 2, 67.
Indiani, C., Santoni, E., Becucci, M., Boffi, A., Fukuyama, K., & Smulevich, G. (2003). New Insight into the peroxidase-hydroxamic acid interaction revealed by the combination of spectroscopic and crystallographic studies. Biochemistry, 42, 14066–14074.
Jiang, X. M., & Lee, H. K. (2004). Solvent bar microextraction. Analytical Chemistry, 76, 5591–5596.
Kaplan, C. D., & Kaplan, J. (2009). Iron acquisition and transcriptional regulation. Chemical Reviews, 109, 4536–4552.
Kozlowski, H., Janicka-Klos, A., Brasun, J., Gaggelli, E., Valensin, D., & Valensin, G. (2009). Copper, iron, and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation). Coordination Chemistry Reviews, 253, 2665–2685.
López-García, I., Rivas, R. E., & Hernández-Córdoba, M. (2012). Hollow fiber based liquid-phase microextraction for the determination of mercury traces in water samples by electrothermal atomic absorption spectrometry. Analytica Chimica Acta, 743, 69–74.
Mashhadizadeh, M. H., Azimi, M. S., Pesteh, M., Sheikhshoaei, I., Ardakani, M. M., & Karimi, M. A. (2008). Flame atomic absorption spectrometric determination of μg amounts of Fe (III) ions after solid phase extraction using modified octadecyl silica membrane disks. Spectrochimica Acta B, 63, 889–892.
Miller, M. J. (1989). Syntheses and therapeutic potential of hydroxamic acid based siderophores and analogs. Chemical Reviews, 89, 1563–1579.
Moghadam, M. R., Shabani, A. M. H., & Dadfarnia, S. (2011). Spectrophotometric determination of iron species using a combination of artificial neural networks and dispersive liquid–liquid microextraction based on solidification of floating organic drop. Journal of Hazardous Materials, 197, 176–182.
Monzyk, B., & Crumbliss, A. L. (1980). Acid dissociation constants (Ka) and their temperature dependencies (AHa, ASa) for a series of carbon- and nitrogen-substituted hydroxamic acids in aqueous solution. Journal of Organic Chemistry, 45, 4670–4675.
Nagai, T., Imai, A., Matsushige, K., Yokoi, K., & Fukushima, T. (2007). Dissolved iron and its speciation in a shallow eutrophic lake and its inflowing rivers. Water Research, 41, 775–784.
Pedersen-Bjergaard, S., & Rasmussen, K. E. (1999). Liquid–liquid–liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Analytical Chemistry, 71, 2650–2656.
Rasmussen, K. E., & Pedersen-Bjergaard, S. (2004). Developments in hollow fiber based liquid-phase microextraction. Trends in Analytical Chemistry, 23, 1–10.
Saçmacı, Ş., & Kartal, Ş. (2008). Selective extraction, separation and speciation of iron in different samples using 4-acetyl-5-methyl-1-phenyl-1-H-pyrazole-3-carboxylic acid. Analytica Chimica Acta, 623, 46–52.
Shemirani, F., & Yousefi, S. R. (2007). Selective extraction and preconcentration of cerium (IV) in water samples by cloud point extraction and determination by inductively coupled plasma optical emission spectrometry. Microchimica Acta, 157, 223–227.
Soylak, M., & Aydin, A. (2011). Determination of some heavy metals in food and environmental samples by flame atomic absorption spectrometry after coprecipitation. Food and Chemical Toxicology, 49, 1242–1248.
Stalikas, C., Fiamegos, Y., Sakkas, V., & Albanis, T. (2009). Developments on chemometric approaches to optimize and evaluate microextraction. Journal of Chromatography A, 1216, 175–189.
Tabrizi, A. B. (2010). Development of a dispersive liquid–liquid microextraction method for iron speciation and determination in different water samples. Journal of Hazardous Materials, 183, 688–693.
Theil, E. C., & Goss, G. J. (2009). Living with iron (and oxygen): questions and answers about iron homeostasis. Chemical Reviews, 109, 4568–4579.
Ullah, N., Tuzen, M., Gul Kazi, T., Citak, D., & Soylak, M. (2013). Pressure-assisted ionic liquid dispersive microextraction of vanadium coupled with electrothermal atomic absorption spectrometry. Journal of Analytical at Spectrometry, 28, 1441–1445.
Vega, F. A., & Weng, L. (2013). Speciation of heavy metals in River Rhine. Water Research, 47, 363–372.
Villar-Navarro, M., Ramos-Payán, M., Fernández-Torres, R., Callejón-Mochón, M., & Bello-López, M. Á. (2013). A novel application of three phase hollow fiber based liquid phase microextraction (HF-LPME) for the HPLC determination of two endocrine disrupting compounds (EDCs), n-octylphenol and n-nonylphenol, in environmental waters. Science of the Total Environment, 443, 1–6.
Wen, X., Deng, Q., Guo, J., & Yang, S. (2011). Ionic liquid-based single drop microextraction of ultra-trace copper in food and water samples before spectrophotometric determination. Spectrochimica Acta A, 79, 1941–1945.
Wu, J., & Boyle, E. A. (1998). Determination of iron in seawater by high-resolution isotope dilution inductively coupled plasma mass spectrometry after Mg(OH)2 coprecipitation. Analytica Chimica Acta, 367, 183–191.
Yaman, M., & Kaya, G. (2005a). Speciation of iron (II) and (III) by using solvent extraction and flame atomic absorption spectrometry. Analytica Chimica Acta, 540, 77–81.
Yaman, M., & Kaya, G. (2005b). Speciation of iron (II) and (III) by using solvent extraction and flame atomic absorption spectrometry. Analytica Chimica Acta, 540, 77–81.
Zeng, C. J., Wen, X. D., Tan, Z. Q., Cai, P. Y., & Hou, X. D. (2010). Hollow fiber supported liquid membrane extraction for ultrasensitive determination of trace lead by portable tungsten coil electrothermal atomic absorption spectrometry. Microchemical Journal, 96, 238–242.
Zeng, C., Lin, Y., Zhou, N., Zheng, J., & Zhang, W. (2012). Room temperature ionic liquids enhanced the speciation of Cr (VI) and Cr (III) by hollow fiber liquid phase microextraction combined with flame atomic absorption spectrometry. Journal of Hazardous Materials, 237, 365–370.
Zhang, Y., Duan, J., He, M., Chen, B., & Hu, B. (2013). Dispersive liquid liquid microextraction combined with electrothermal vaporization inductively coupled plasma mass spectrometry for the speciation of inorganic selenium in environmental water samples. Talanta, 115, 730–736.
Zhao, L., Zhong, S., Fang, K., Qian, Z., & Chen, J. (2012). Determination of cadmium(II), cobalt(II), nickel(II), lead(II), zinc(II), and copper(II) in water samples using dual-cloud point extraction and inductively coupled plasma emission spectrometry. Journal of Hazardous Materials, 239, 206–212.
Acknowledgments
The authors would like to thank Semnan University Research Council for financial support of this work. The authors also would like to especially thank Miss S. Sabzalian, Department of Chemistry, Semnan University, Semnan, Iran.
Conflict of interest
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saeidi, I., Barfi, B., Asghari, A. et al. Ionic-liquid-based hollow-fiber liquid-phase microextraction method combined with hybrid artificial neural network-genetic algorithm for speciation and optimized determination of ferro and ferric in environmental water samples. Environ Monit Assess 187, 631 (2015). https://doi.org/10.1007/s10661-015-4860-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4860-4