Abstract
Bacteria from the genera Dickeya and Pectobacterium, the causative agents of soft rot and blackleg, trigger significant economic losses in potato production worldwide. Efficient struggle with these phytopathogens is highly challenging taking into consideration the lack of available control procedures. As only preventive measures are accessible, we decided to provide insight into the soft rot Pectobacteriaceae (SRP) present in Poland. During the growing seasons of 2013 and 2014, altogether 531 potato plants were collected from 138 seed potato fields and 23 storage facilities. Plant origin of the isolated bacteria, frequencies of coinfections with different species, the affected potato cultivars in addition to seasonal variation in the occurrence of SRP were studied. It was shown that bacteria from the Pectobacterium genus were abundant and outnumbered the ones classified to Dickeya spp. The vast majority of strains was isolated from the plant samples collected in July 2013 or in June–July 2014. The presence of all taxa of interest: Pectobacterium atrosepticum, Pectobacterium carotovorum, Pectobacterium parmentieri, Dickeya dianthicola and Dickeya solani were confirmed in July each year. We were able to isolate bacteria from the genus Dickeya and Pectobacterium from 35 out of 58 potato cultivars tested. The majority of SRP was isolated from potato stems, not from potato tubers. In four cases, coinfections of potato samples with even three diverse species of SRP, i.e. P. atrosepticum, P. carotovorum and P. parmentieri, were noted. It seems that since the first documented appearance of Dickeya solani in Poland in 2005, this pathogen has not played a dominating role in our country. The reported data describing the appearance and distribution of SRP in Poland might allow for prediction of the risks associated with infections initiated by these bacteria.
Similar content being viewed by others
Introduction
Dickeya and Pectobacterium spp. are the causative agents of blackleg of potato and soft rot on various plant species. These phytopathogenic bacteria underwent numerous taxonomic reclassifications and were designated to miscellaneous species, genera and families (Gardan et al. 2003; Samson et al. 2005). Previously, they had been referred to as soft-rot Enterobacteriaceae or pectinolytic erwinias (Perombelon and Kelman 1980). However, due to recent revision in their phylogeny based on the sequences of 1548 core proteins and 16S rDNA, Dickeya and Pectobacterium spp. belong now to a newly established Pectobacteriaceae family (Adeolu et al. 2016). Therefore, the term soft-rot Pectobacter-iaceae (SRP) seems suitable with reference to these disease-causing bacteria (Motyka et al. 2017).
SRP exhibit a rather broad host range as they are capable of infecting at least 35% of angiosperm plant orders (Ma et al. 2007) including crops, vegetables and ornamentals e.g. potato, tomato, maize, cabbage, beetroot, chicory, pepper, carrot, chrysanthemum or saintpaulia (African violet). Potato plants gained special attention as they belong to the top five crops (after sugar cane, maize, rice and wheat) with a rising production trend from 327,600 Kt in 2000 to 368,096 Kt in 2013 (Food and Agriculture Organization of the United Nations (FAO), 2015). A large scale potato cultivation started in the eighteenth century and up to 1970 Poland produced 50 million tonnes of potatoes a year, being the 2nd largest producer in the world, following the Soviet Union (Zarzecka 2009). Currently, Poland is the 7th producer of potato worldwide behind China, India, Russia, Ukraine, USA and Germany (FAO 2012).
A high significance of Dickeya and Pectobacterium spp. was underlined by Mansfield et al. (2012) as they were listed on positions 9th and 10th, respectively, among the top ten plant pathogenic bacteria. It is challenging, however, to assess the worldwide economic impact of SRP on potato production, especially taking into consideration the common occurrence of these pathogens in diverse geographical zones, variable crop pricing and lack of uniform seed certification policies in different countries (Motyka et al. 2017; Toth et al. 2011). Additional obstacles are associated with the fact that Dickeya and Pectobacterium cause losses in the fields, during harvest, storage and marketing of the crop (Perombelon 2002). Over 40 years ago, Perombelon and Kelman (1980) estimated the total economic damage associated with SRP to be 50–100 × 106 US dollars annually on a worldwide basis. More recent data originate from the Netherlands, where direct losses connected with rejections and downgrading of seed tubers amount to 30 million euros a year (Toth et al. 2011).
Blackleg and soft rot are considered seed-borne diseases. However, Dickeya and Pectobacterium spp., aside from spreading from the affected mother tuber (latently infected seed potato), are capable of penetrating plant or tuber tissue through natural openings (stomata and lenticels) or wounds. Also, the contaminated surface or irrigation waters, soil, plant remains, insects, nematodes or agricultural machines not meeting hygiene standards can be a source of infection (Cappaert et al. 1988; Nykyri et al. 2014; Rossmann et al. 2018; van der Wolf and Kastelein 2014). Once virulent strains reach the vulnerable host under disease-promoting environmental conditions (Barrett et al. 2009), typical SRP-triggered syndromes develop (Perombelon and Kelman 1980). Soft rot disease involves water-soaked, slimy maceration of the inner potato tuber tissue, while blackleg is associated with decay of the stem base followed by chlorosis, wilting leaves and lack of progeny tubers (Perombelon 2002). These outcomes result mainly from the action of plant cell wall degrading enzymes i.e. pectinases, cellulases and proteases (Hugouvieux-Cotte-Pattat et al. 1996).
Currently, no control methods have been successfully implemented in order to eradicate Dickeya and Pectobacterium spp. after infecting susceptible crops (Toth et al. 2011). Therefore, the only preventive measures routinely applied to limit the spread of SRP are regular controls of seed plantations and removal of the infected plants. Our group has been monitoring the presence of SRP on seed potato fields of Poland since 1996. At that time, Pectobacterium atrosepticum and Pectobacterium carotovorum species turned out to be widespread (Sledz et al. 2000; Waleron et al. 2002). Our studies that followed indicated also common presence of Pectobacterium parmentieri (earlier Pectobaterium wasabiae) on potato plantation (Waleron et al. 2013; Motyka et al. 2017; Zoledowska et al. 2018b). The first appearance of Dickeya solani in Poland was noted during the routine testing of seed potato plantation in 2005 (Slawiak et al. 2009). According to our data, solely D. solani and Dickeya dianthicola have been isolated from potato plant/tuber samples in Poland, while Dickeya zeae and Dickeya chrysanthemi were detected in the investigated surface water samples (Potrykus et al. 2016). Finally, our special attention was driven to the recently established species, namely D. solani (van der Wolf et al. 2014) and P. parmentieri (Khayi et al. 2016). In spite of significant genomic homogeneity among the studied D. solani isolates, we showed big differences in their virulence (Potrykus et al. 2016; Golanowska et al. 2017, 2018). On the contrary, a high divergence in the virulence factors production, correlating with variations in the genomic structure, has been revealed in P. parmentieri strains (Zoledowska et al. 2018a, 2018b). It is worth noting that P. parmentieri strains formed a significant group within the SRP population in Poland already in 1996, though, being misclassified to Pectobacterium carotovorum subsp. carotovorum, they remained unnoticed (Waleron et al. 2013).
As our former studies mainly focused on phenotypic and genotypic characterisation of solely D. solani and P. parmentieri (Golanowska et al. 2017, 2018; Potrykus et al. 2016; Zoledowska et al. 2018a, b), in this work we analysed the prevalence of various groups of Dickeya and Pectobacterium spp. on seed potato plantations in Poland in the years 2013 and 2014. In more detail, the sources of SRP, seasonal variation in their occurrence, and the type of fertilizing practices applied to the seed potato fields yielding Dickeya or Pectobacterium isolates were investigated. The collected data may contribute to the description of environmental factors favouring infections of plants with different SRP.
Materials and methods
Plant material
Altogether, 531 samples of potato plants with the symptoms of blackleg and/or soft rot, soft rot-affected potato tubers and asymptomatic weeds accompanying the diseased potato plants were collected in Poland from 138 seed potato plantations (from May to September) or from 23 storage facilities (from October till December), mainly by the inspectors of Regional Inspectorates of Plant Health and Seed Inspection Service in Poland (RIPH & SIS) in the years 2013 and 2014. Also, several of these samples were acquired from the collaborating institutions: Institute of Plant Breeding and Acclimatization - Jadwisin and Mlochow Research Centers, Potato Breeding Zamarte, Plant Breeding in Szyldak and Pomeranian-Mazurian Potato Breeding in Strzekecino. Together with the plant tissue provided in separate string bags to the Laboratory of Plant Protection and Biotechnology of the Intercollegiate Faculty of Biotechnology University of Gdansk and Medical University of Gdansk (IFB UG & MUG), the inspectors or cooperating growers attached detailed surveys on the geographical origin and collection date of the samples, the planted potato variety and the fertilizing methods applied to the corresponding field as described by Potrykus et al. (2016).
Altogether, 248 plant samples from 2013 (124 potato stems, 74 potato tubers, and 50 accompanying weeds; Table 1) and 283 from 2014 (153 potato stems, 87 potato tubers, and 43 weeds; Table 1) were subjected to analysis. Material from 58 diverse potato varieties was sampled. The plant samples were collected from 13 voivodeships in 2013 and 12 in 2014 (out of 16 in Poland; Supp. Figure 1).
Isolation and to-species identification of SRP
SRP were isolated as described previously by Potrykus et al. (2016) and Zoledowska et al. (2018b). Briefly, 1 g of the sampled plant material was suspended in 10 ml of 50 mM phosphate buffer pH 7.2 and subsequently homogenized with a hand homogenizer in a universal 12 × 15 cm extraction bag (Bioreba, Switzerland). The resultant plant homogenate was serially diluted to 10−6 in sterile 0.85% NaCl and then 100 μl of the 10−5 and 10−6 dilutions were plated on two Crystal Violet Pectate (CVP) plates (Hélias et al. 2012). Subsequently, the CVP plates were incubated for 48 h at 28 °C. If no bacterial growth was observed, the plating procedure was repeated with less diluted plant homogenates. Bacterial colonies that formed characteristic cavities were collected and replated in a reductive manner on CVP and TSA media until the axenic culture state was reached. All isolates were subjected to multiplex PCR-based identification (Potrykus et al. 2014). At first, bacterial lysates were prepared by freezing the biomass of one colony per strain in 200 μl of distilled water at −20 °C for 20 min. Then, the multiplex PCR-reaction was performed as described previously by Potrykus et al. (2014). P. atrosepticum strains were assigned to the species level basing on the acquisition of a specific amplicon of 439 bp with the Y45 and Y46 primers (Frechon et al. 1998; Potrykus et al. 2014). Dickeya spp. strains were identified by amplification of a specific DNA fragment of 133 bp with the use of Df and Dr starters (Laurila et al. 2008; Potrykus et al. 2014). In order to identify Dickeya strains to the species level we analysed the dnaX gene sequence and performed the rep-PCR according to Potrykus et al. The strains yielding PCR amplicons with the ExpccF and ExpccR primers (550 bp; Kang et al. 2003; Potrykus et al. 2014) in the multiplex PCR reaction were further tested with a P. parmentieri-specific single PCR reaction accordingly to De Boer et al. (2012). The strains that gave specific amplicons with PhF and PhR primers (De Boer et al. 2012) were attributed to the P. parmentieri species, while the ones that yielded specific amplicons just with ExpccF and ExpccR starters and negative in the P. parmentieri-specific test were assigned to P. carotovorum species. Electrophoretic separation (100 V, 40 min) of the PCR amplicons was conducted in 1.5% agarose gel in 0.5 x Tris Borate EDTA (TBE) buffer. 0.5 mg l−1 ethidium bromide was applied for DNA staining prior to washing and visualization under UV light with a ChemiDoc XRS system (Bio-Rad, USA). In the performed PCR assays, the following reference strains originating from international bacterial collections were used: D. solani IFB0099, P. atrosepticum SCRI 1086, P. carotovorum subsp. carotovorum SCRI 136, P. parmentieri SCC3193. Full genomic sequences of 14 SRP strains (12 of P. parmentieri and two of D. solani) isolated in this study are available in the Genbank database under the following accession numbers: GCA_003992745.1, GCA_003628655.1, GCA_003628675.1, GCA_003628575.1, GCA_003628635.1, GCA_003628735.1, GCA_003628595.1, GCA_003628695.1, GCA_003628615.1, GCA_003628715.1, GCA_003628015.1, GCA_003628025.1, JABAOP000000000 and JABAOQ000000000 (Zoledowska et al. 2018a; Motyka-Pomagruk et al. 2020).
Data analysis and visualization
Data from the surveys describing the collected plant samples together with the frequencies of distinct SRP isolations were summarized, analysed and plotted with the use of Microsoft Excel 365 software (Microsoft Corporation, USA). The final high resolution figures in addition to Venn diagrams were prepared and assembled with the use of Inkscape software (version 0.92, GNU General Public License).
Results
Abundance of SRP on the territory of Poland
In the years 2013 and 2014, 101 and 124 strains belonging to SRP (Table 1) were isolated from the investigated plant samples, respectively. Therefore, excluding the noted coinfection events, 73 out of 248 (29%) in addition to 106 out of 283 (37%) of the plant samples collected in 2013 and 2014 harboured SRP. Notably, SRP strains were detected on the majority of Polish territory, namely in 11 regions out of 13 or 12 included in the 2013 or 2014 study, respectively (Supp. Figure 1). The Pectobacterium spp. isolation events (94 in 2013 and 114 in 2014; Table 1) turned out to outnumber the ones yielding Dickeya spp. strains (seven in 2013 and 10 in 2014; Table 1). In both years a similar number of D. solani (4 and 5) and D. dianthicola (3 and 5) strains was isolated. Pectobacterium spp. were widespread on the territory of Poland (their occurrence was noted in at least 11 Polish regions; Supp. Figure 1), while the presence of Dickeya spp. was restricted solely to four regions in each year of the study (Supp. Figure 1).
The most frequently isolated SRP species in 2013 was P. carotovorum (36% of the total number of collected strains), followed by P. parmentieri (32%), P. atrosepticum (26%) and Dickeya spp. (7%) (Fig. 1a). In 2014, the dominating trends were similar, however this time P. atrosepticum was isolated more commonly (38%) in comparison to P. carotovorum (31%), P. parmentieri (23%) and Dickeya spp. (8%) (Fig. 1a). In Fig. 1b, the outcomes of the former studies conducted by our group in 1996, 2005 and 2011 are depicted. It is worth noting that P. carotovorum is presented together with P. parmentieri as the identification method utilized at that time did not differentiate these two species. Notably, both in 1996 and 2011, P. carotovorum together with P. parmentieri constituted the most abundant SRP group, while P. atrosepticum dominated in 2005 (Fig. 1b). Considering all the data, Pectobacterium spp. strains were the leading cause of potato soft rot and blackleg in Poland from 1996 to 2014 contrary to Dickeya spp., whose population was minor and rather stable (Fig. 1) since the first report on the occurrence of Dickeya spp. in our country in 2005 (Slawiak et al. 2009).
The most frequently isolated groups of soft rot Pectobacteriaceae (SRP) during long-term monitoring of seed potato plantations in Polanda Prevalence of distinct Pectobacteriaceae revealed within the herein described study of 2013 and 2014. b The dominating species from the Pectobacteriaceae family according to the previous surveys from years 1996, 2005 and 2011. From the lightest tint:  – Dickeya spp. (Dsp),
– Dickeya spp. (Dsp),  – P. parmentieri (Ppa),
– P. parmentieri (Ppa),  – P. atrosepticum (Pba),
– P. atrosepticum (Pba),  – P. carotovorum (Pc). In the years 1996, 2005 and 2011, Pc was not distinguished from Ppa, therefore the occurrence of these two species is depicted together in a stripped pattern -
– P. carotovorum (Pc). In the years 1996, 2005 and 2011, Pc was not distinguished from Ppa, therefore the occurrence of these two species is depicted together in a stripped pattern - 
Seasonal variation in the occurrence of SRP
Figure 2a shows the percentage distribution of plant samples collected by the RIPH & SIS inspectors or specialists from the collaborating institutions from seed potato fields in Poland during each month of the study. The majority of the tested plants were sampled in July. Interestingly, plant samples collected in August and September of 2013 yielded higher percentages of SRP isolates (19% and 4%; Fig. 2b) than it was expected from their abundance (10% and 1%, respectively; Fig. 2a). We made similar observations in case of the samples picked up from the fields in June 2014 (31% of total plants investigated that year), which were proven to harbour 48% of SRP (Fig. 2a, b).
Months in which the strains of SRP were isolated from seed potato fields in Poland during 2013 and 2014 growing seasons. a Months of plant samples collection. b Months of SRP isolation. c Months of isolation of the distinct Dickeya and Pectobacterium spp. strains. The included months starting from the lightest tint: May –  , June –
, June –  , July –
, July –  , August –
, August –  , September –
, September –  . Solely samples that originated from potato fields (collected from May until September), not the ones picked from storage facilities, are shown
. Solely samples that originated from potato fields (collected from May until September), not the ones picked from storage facilities, are shown
Focusing on distinct SRP, a higher interdependence between the plant collection period and the year of study rather than the SRP group involved was noted (Fig. 2 ac). The monthly distribution of P. carotovorum isolates in 2013 and 2014 reflected the observed partitioning among the total number of SRP stated for the corresponding year (Fig. 2bc). A similar remark regarded P. parmentieri isolates in 2013, however in 2014 the highest percentage of strains belonging to this species (59%) originated from the plants collected in June, which did not represent the highest fraction of the total samples tested (31%) (Fig. 2 ac). Of the SRP species investigated, the monthly distribution of P. atrosepticum isolates was standing out from the frequencies of plant samples collections and the total SRP isolations. P. atrosepticum was not only a dominating species in potato fields in 2014, but also most of these strains (96% in 2013 and 100% in 2014) were isolated at the beginning of summer i.e. June or July (Fig. 2c). As shown in Fig. 2 ac, months in which Dickeya spp. were isolated did not relate with the abundance of the received plant samples, but the low number of the obtained Dickeya spp. strains (Table 1) needs to be considered. Interestingly, during both years of study, July turned out to be the only month in which the presence of bacteria from all the analysed taxons, namely P. atrosepticum, P. carotovorum, P. parmentieri and Dickeya spp., was confirmed on seed potato fields in Poland from 2013 to 2014 (Table 1, Fig. 2c).
Beside the diseased potato stems and tubers in addition to the weed samples collected from the fields, symptomatic potato tubers (17 from four regions in 2013 and 12 samples from five voivodeships in 2014; Table 1) originating from storage facilities (collected from October until December) were also tested for the presence of SRP with in the frames of this research. Interestingly, no Dickeya spp. were isolated from potato tubers of such origin in contrast to P. atrosepticum, P. carotovorum or P. parmentieri, which were acquired from some of these samples (2, 6 and 6, respectively; Table 1) during the 2-year study.
SRP coinfection events
From 26 plant samples collected in 2013, more than one SRP species was isolated (Fig. 3a). Coinfection of P. carotovorum with P. parmentieri occurred most often in 2013 (13 cases; Fig. 3a). Notably, two cases were recorded in which three diverse pathogens, namely P. parmentieri, together with P. carotovorum and P. atrosepticum, were isolated from the same plant sample (Fig. 3a). In 2014, 16 coinfection events were detected (Fig. 3b). Similarly to the year 2013, most common coinfections in 2014 involved also P. parmentieri and P. carotovorum (four cases; Fig. 3b). Analogously to 2013, there were two plant samples collected in 2014 that yielded three diverse SRP species (Fig. 3b). It is worth noting that the SRP species co-occurrence pattern is exactly the same throughout the years of study, in other words, each year the same species either were detected together or were not present in such a combination (Fig. 3). Noteworthily, no P. atrosepticum strain coinfected any of the tested plants together with Dickeya spp. It also seems interesting that in each year of study, there was one weed sample that was noted to harbour multiple pathogens (either P. carotovorum and P. atrosepticum in 2013 or P. parmentieri and P. atrosepticum in 2014).
Coinfection events with Dickeya and Pectobacterium spp. isolates in 2013 (a) and 2014 (b). Numbers referring to the number of isolates classified to certain species of Pectobacteriaceae are enclosed within the ellipses colored in  for P. carotovorum (Pc), in
for P. carotovorum (Pc), in  for P. atrosepticum (Pba), in
for P. atrosepticum (Pba), in  for P. parmentieri (Ppa) and in
for P. parmentieri (Ppa) and in  for Dickeya spp. (Dsp). Numbers presented in bold within the common regions of the ellipses correspond to the number of coinfection events with the strains belonging to the Pectobacteriaceae species marked accordingly to the above-described coloration
for Dickeya spp. (Dsp). Numbers presented in bold within the common regions of the ellipses correspond to the number of coinfection events with the strains belonging to the Pectobacteriaceae species marked accordingly to the above-described coloration
Plant origin of SRP strains
In 2013 and 2014, the RIPH & SIS Inspectors and other specialists from the collaborating institutions collected the highest number of blackleg- and soft rot-affected samples of potato stems (124 and 153, respectively), followed by the diseased potato tubers (74 and 87) and the asymptomatic accompanying weeds (50 and 43) (Table 1). In this view, it does not seem surprising that in 2013 most SRP strains were isolated from potato stems (61%), a significantly lower number from potato tubers (33%), while solely several strains from the accompanying weeds (6%) (Fig. 4a, b). A similar observation concerned the year 2014, namely 73% of SRP isolates originated from potato stems, 20% from potato tubers, whereas 7% were acquired from the weeds (Fig. 4b). Interestingly, in both years of study, SRP strains were isolated from potato stems more frequently (61% and 73%) than it was expected basing on the number of the obtained samples of such material (50% and 54%, respectively; Fig. 4a, b). The accompanying weeds were revealed to be the environmental source of SRP significantly less often (6% and 7%) than it was predicted (20% and 15%, respectively; Fig. 4a, b). The abundance of diverse SRP groups in different types of the studied plant material is shown in Fig. 5. We observed that the distribution of the studied species in potato stems and tubers (Fig. 5) resembles the pattern of the general prevalence of distinct SRP in all the studied plant material in the corresponding years 2013 and 2014 (Fig. 1a). Focusing on the analysed accompanying weed samples, we isolated most frequently either P. parmentieri or P. atrosepticum (6 isolates of each in 2013–2014) from such material (Table 1). Curiously, no Dickeya spp. were acquired from the weed samples tested (Table 1; Fig. 5).
Plant or plant organ origins of SRP isolated in 2013 and 2014. Distribution of the plant samples tested in 2013 and 2014 (a) was juxtaposed to the percentages of different sample types yielding the Pectobacteriaceae isolates (b).  corresponds to potato tubers,
corresponds to potato tubers,  refers to potato stems, while
refers to potato stems, while  shows weed samples
shows weed samples
Altogether, SRP strains were isolated from 35 potato cultivars out of 58 investigated (Fig. 6). The SRP-affected potato cultivars represented miscellaneous cooking qualities: AB (Denar, Lord and Vineta), B (Irga, Satina, Bellarosa and Jelly) or B-BC (Tajfun and Owacja), both starch and table potatoes (Fig. 6) in addition to early season (Denar, Miłek, Lord) or late-season (Jelly, Bryza, Gustaw) varieties (Nowacki 2012). 21 diverse potato cultivars were affected by P. carotovorum strains (Fig. 6). A lower number (19) of the analysed cultivars yielded either P. atrosepticum or P. parmentieri isolates (Fig. 6). The less frequently acquired Dickeya spp. strains were obtained just from 10 of the investigated potato cultivars (Fig. 6). Interestingly, all the analysed Dickeya and Pectobacterium spp. were isolated from Irga, Satina, Vineta and Bellarosa cultivars (Fig. 6), while Jelly, Denar, Harpun, Lord, Owacja, Kuba, Cyprian and Tajfun cultivars were the environmental source of different Pectobacterium species of interest (Fig. 6).
Potato cultivars collected in 2013 and 2014 that harbored the isolated Pectobacteriaceae strains. Potato cultivars from which the Pectobacteriaceae isolates were obtained are enclosed within the ellipses colored, starting from the left and from the darkest tint: in  for P. carotovorum (Pc), in
for P. carotovorum (Pc), in  for P. atrosepticum (Pba), in
for P. atrosepticum (Pba), in  for Pectobacterium parmentieri (Ppa) and in
for Pectobacterium parmentieri (Ppa) and in  for Dickeya spp. (Dsp). More than one Pectobacteriaceae strain was acquired from the cultivars presented in the common regions of the ellipses. Starch potatoes are italicized, while the table potatoes are depicted in the normal font
for Dickeya spp. (Dsp). More than one Pectobacteriaceae strain was acquired from the cultivars presented in the common regions of the ellipses. Starch potatoes are italicized, while the table potatoes are depicted in the normal font
Fertilizing methods applied to plants infected with SRP strains
We also analysed the distribution of the applied fertilizing practices to potato fields from which the investigated plant samples had been collected (Table 1). In 2013, 100 of the plants included in this study (Table 1) were fertilized with commercial mixtures of nitrogen, phosphorous, and potassium complexes, i.e. inorganic NPK fertilizers. In addition, 45 of the plants were subjected to treatment with solely organic fertilizers such as manure, compost, slurry, after-crop, natural urea or any other referred to as ‘ecological’ (Table 1). Besides, NPK fertilizers with organic additives concerned 40 (Table 1) of the tested plants. Also, the NPK mixture with mineral supplements, in this case usually secondary minerals (Ca, S or Mg) applied for pH adjustment in liming materials or micronutrients (e.g. Zn, Cl, B, Mo, Cu, Fe, Mn, Co or Ni), was commonly implemented (38 plants). Last but not least, most complex mixtures of NPK fertilizers supplemented with both organic and mineral additives concerned 16 of the fields studied in 2013 (Table 1). Similarly in 2014, implementation of just NPK fertilizers was most common (75 of the obtained plants), followed by organics (56 plants), NPK supplemented with organics (41 plants), NPK with additional minerals (37 plants) and NPK enriched with both organic and mineral supplements (25 plants) (Table 1).
The detailed information concerning samples from which a distinct SRP species was acquired in a stated year is shown in Table 1. It is worth noticing that during both years of study approx. 50% of plant samples yielding P. atrosepticum isolates were treated together with organic fertilizers or NPK supplemented with organics (Table 1). Concerning P. carotovorum strains, they were predominantly isolated in 2013 from the fields subjected to rich fertilization with NPK supplemented with organics (36%), which diverged from the overall fertilizing pattern applied to potato fields included in 2013 (Table 1). Also in 2014, this fertilization method was overrepresented (21% vs. 14%; Table 1) among P. carotovorum-yielding plant samples. In the case of Dickeya spp. and P. parmentieri, the majority of plant samples from which these isolates were acquired underwent NPK fertilizing, exceeding the frequency of this treatment utilization each year (Table 1).
Discussion
The first scientific reports on blackleg and soft rot diseases affecting potato production in Europe date back to 1972 (Naumann and Zielke 1977). Despite significant improvements allowing for instance the detection of distinct SRP species responsible for a given disease outbreak, still significant economic losses result from the presence of bacteria from the genera Pectobacterium and Dickeya in potato fields, especially taking into account a high percentage of latent infections amounting even to 2–30% under intensive potato production (Toth et al. 2011). Therefore in this work, we investigated the prevalence of Pectobacterium and Dickeya spp. on seed potato fields in Poland with a focus on the dominating SRP species in 2013 and 2014 growing seasons. This knowledge supported our long-term monitoring studies, which revealed that Pectobacterium spp. in contrast to Dickeya spp. was the leading cause of blackleg and soft rot diseases on seed potato fields in Poland from 1996 to 2014 (Dees et al. 2017; Golanowska et al. 2017; Potrykus et al. 2016; Slawiak et al. 2013; Sledz et al. 2000; Waleron et al. 2002; Zoledowska et al. 2018b).
As herein reported, P. atrosepticum turned out to be one of the most frequently isolated SRP species in Poland. Isolates classified to this species were not only most prevalent in 2014 (38%), but also formed a significant fraction of SRP strains in 2013 (26%). High importance of P. atrosepticum isolates in our country was already indicated by Sledz et al. (2000). The predominance of P. atrosepticum in temperate climate regions was stated over 35 years ago by Perombelon and Kelman (1980). This species is widely present in many potato growing regions, for instance in Scotland (Toth et al. 2011), Finland (Laurila et al. 2008), Turkey (Ozturk et al. 2018), USA (especially Colorado, Charkowski 2018) or Canada (De Boer et al. 2012).
Our research showed that the dominating SRP species in Poland changed throughout the years of study. P. carotovorum dominated in the screened regions in 2013 (36%), which is in line with the previously reported outcomes on notable contribution of this species to potato decay under temperate climate conditions (de Haan et al. 2008; Toth et al. 2011). For example in the Netherlands, all the investigated blackleg incidences in 1987, 1989, 1993, 2001 and 2005 were associated with the occurrence of P. carotovorum (van Beckhoven et al. 2001). Similarly in France, solely P. carotovorum was identified in approx. 20% of blackleg-diseased plants (after de Haan et al. 2008). Even in cooler geographical regions like Finland, a high abundance of P. carotovorum strains was noted (Pasanen et al. 2013). In Norway, both P. atrosepticum and P. carotovorum were frequently detected on potato fields, however the other commonly isolated SRP species in Poland (32% and 23% in 2013 and 2014, respectively) P. parmentieri, was acquired in that country just in a single case (Dees et al. 2017).
It is worth considering that there was a specific group of P. carotovorum subsp. carotovorum strains, which included highly virulent P. c. subsp. carotovorum isolated in the Netherlands (vPcc; de Haan et al. 2008) and virulent P. carotovorum subsp. carotovorum isolated in Poland (Slawiak et al. 2013). These strains adhered earlier to the recA PCR-RFLP group 3 Pcc (Waleron et al. 2002). The reclassification of P. carotovorum SCC3193 to P. wasabiae SCC3193 (Nykyri et al. 2012) initiated changes in the taxonomy of other P. carotovorum isolates. A large group of P. carotovorum strains isolated in Poland were reclassified at first to P. wasabiae (Slawiak et al. 2013; Waleron et al. 2013) and then to P. parmentieri (Khayi et al. 2016). To this day, P. parmentieri has been reported to contribute to the SRP populations of Canada, Germany, Ireland, Finland, France, Poland, Scotland, USA, Turkey, Spain andthe Netherlands among others (De Boer et al. 2012; Ge et al. 2018; Nabhan et al. 2012; Nykyri et al. 2012; Ozturk et al. 2016; Suárez et al. 2017; Waleron et al. 2013). With reference to the recentdetection of Pectobacterium brasilience in Poland (Babinska and Waleron, personal communication), our study will be enriched in the future by monitoring of thepresence of this species on seed potato fields in our country. This effort is motivated by the current data indicating the growing impact of this species on European potato production (Nunes Leite et al. 2014; van der Wolf et al. 2017).
In the herein presented work we reported low abundance of D. dianthicola and D. solani isolates in Poland. A few years ago, D. solani was believed to be highly prevalent in Europe (Cahill et al. 2010; Degefu et al. 2013; Helias 2006; Laurila et al. 2008; Palacio-Bielsa et al. 2006; Toth et al. 2011; van der Wolf et al. 2014). The studies of the Norsk Institutt for Bioøkonomi also support the current minor significance of this species in the development of soft rot or blackleg disease outbreaks in comparison to the other members of SRP (Dees et al. 2017).
The encountered weather conditions influence to a high extent blackleg- or soft rot- related disease severity (Perombelon and Kelman 1980). The average air temperature and insolation in Poland in 2014 was higher than in 2013, however, lower precipitation was noted (Institute of Meteorology and Water Management - National Research Institute, Poland). As suggested by Perombelon (2002) and Toth et al. (2011) the total incidence of blackleg (pre- and post- emergence) tends to increase together with an elevated soil temperature and moisture if the pathogen inoculum is low. As throughout each year of study only one of the above-listed disease-promoting factors occurred, this might be the reason explaining the collection of similar numbers of blackleg- and soft rot-affected plants or the comparable quantities of the isolated SRP in 2013 and 2014.
Higher air temperatures in 2014 were reflected in the percentage distribution throughout the months in which the diseased plant samples were collected, meaning that a greater number of blackleg- or soft rot-affected plants was acquired from the fields earlier (already in June) in 2014 than in 2013. Nearly all P. atrosepticum strains were obtained in early summer i.e. in June or July, which is the most favourable period for blackleg symptoms development, as reported before (de Haan et al. 2008). Considering better adaptation of P. atrosepticum to lower temperatures, the corresponding disease symptoms might be the outcome of earlier infections in comparison to these caused by the other SRP species. Also in Japan, a seasonal variation in the occurrence of P. atrosepticum in potato fields was noted – these strains were abundant in early summer and fall, contrary to the hot season of midsummer (Tsuyama and Sakamoto 1952). Interestingly, in the current research the members of all SRP species were detected on potato fields in Poland in July during both years of study. This observation correlates with the earlier reports of Powelson (1980), who noted the highest seasonal blackleg and soft rot incidence throughout July, while testing potato fields in Columbia Basin of Oregon.
In this work, more SRP strains were obtained from potato stems than tubers. It is worth noting that the RIPH & SIS inspectors provided us with a higher number of stem samples than these of tubers, however normalization of the number of successful SRP isolations to the number of samples tested would lead also to the above-presented conclusion. Likewise, De Boer et al. (2012) provided proofs for obtaining a greater number of SRP from potato stems than tubers.
In spite of the plants and tubers collected from the fields, we searched for SRP in potato tubers acquired from October to December from the storage facilities. All species of the interest from the genus Pectobacterium were isolated from this material, however, no strains belonging to the Dickeya genus were acquired from these samples. There were previous reports on the isolation of Pectobacterium spp. strains from potatoes of the warehouse origin (Perombelon 2002; Toth et al. 2011).
In our research, the isolates of all the included Pectobacterium species, contrary to Dickeya spp., were acquired from the accompanying weeds tested. By now, little is known about the alternative plant hosts of Dickeya spp. For instance, this pathogen was isolated in Sweden from Solanum dulcamara (Olsson 1985), perennial herbaceous weed growing in lowlands in proximity to potato fields (Toth et al. 2011). In Poland, solely P. c. subsp. carotovorum was isolated from the naturally occurring S. dulcamara (Fikowicz-Krosko et al. 2017). The fact of successful isolations of P. carotovorum from the investigated asymptomatic weed samples might be linked with a wide host range of this pathogen. For instance, P. carotovorum strains were obtained earlier from Tagetes patula, Arctium minus, Cichorium intybus, Brassica oleracea, Zantedeschia aethiopica, Ornithogalum dubium, Capsicum annuum, Solanum esculentum, Cucumis melo, Lilium spp., Hippeastrum spp., Schismatoglottis spp. and Ornithogalum saundersiae (Ma et al. 2007; Yishay et al. 2008). It is interesting to notice that contrary to Ma et al. (2007), who described the host range of P. atrosepticum as mostly limited to potato, we did isolate 6 P. atrosepticum strains from the accompanying weeds. Also six P. parmentieri strains were isolated in this research from such material. Interestingly, other groups acquired P. parmentieri strains from e.g. sweet pepper (Waleron et al. 2013), Solanum melongena, Solanum lycopersicum, Ipomoea batatas or Brassica rapa (Golkhandan et al. 2013). Nonetheless, due to the recent separation of P. parmentieri from P. wasabiae species (Khayi et al. 2016), there is still an ongoing discussion on the alternative hosts reported for each of these taxa.
Focusing on potato, currently there are 114 diverse potato cultivars (64 domestic and 50 foreign) catalogued in the Polish registry of potato varieties (Stypa 2015). We obtained soft rot bacteria from the samples of 35 cultivars from 58 collected in this study. The most commonly grown table potato cultivar in Poland with 9.5% of the total acreage, namely Vineta (Stypa 2015), was the environmental source of all four taxa of SRP. Second in line are Denar, Lord and Tajfun from which we collected bacterial isolates from all three Pectobacterium spp. of interest. It is worth mentioning that from the cultivars Arielle, Igor, Inovator and Zuzanna only strains of D. dianthicola and D. solani but not those belonging to the genus Pectobacterium were isilated. Concerning the most common cultivar intended for industrial processing, namely Innovator (9.1% of market share; Stypa 2015), it yielded solely Dickeya spp. isolates. As regards the starch variety, named Kuba, of the greatest market share (1.7%; Stypa 2015), all the studied Pectobacterium spp. were acquired from the analysed samples. Former studies on the appearance of SRP on different potato cultivars in Poland are scarce. However, Kapsa and Gawinska-Urbanowicz (2013) reported a high (25%) disease incidence caused by Pectobacterium spp. on potato cv. Owacja in the Pomeranian region.
In addition, we noted that coinfections with diverse SRP occurred quite often i.e. out of 101 or 124 strains collected in 2013 and 2014, respectively, either 54 or 34 isolates originated from plant samples harbouring more than a single SRP species. It indicated that in 2013 about 50% of strains were isolated from potato plants coinfected with at least 2 bacterial species. In contrast, in 2014 only about 27% of strains were acquired from coinfected plants. Both in 2013 and 2014 the most common co-existence was observedamong P. carotovorum and P. parmentieri. However in 2014, this most frequent type of coinfection took place 3 three times less often than in 2013, even if the number of P. carotovorum and P. parmentieri strains isolated both years was similar. Previously, Gross et al. (1991) and Sledz et al. (2000) showed that P. carotovorum subsp. carotovorum strains were present in the same potato field as the isolates of P. atrosepticum. Kim et al. (2009) revealed that not only multiple Pectobacterium species were encountered in one field, but also sporadically, P. carotovorum and P. wasabiae (currently P. parmentieri) were detected in the same symptomatic tuber. Besides, we noticed the lack of Dickeya spp. and P. atrosepticum coinfections, which might be associated with diverse temperature optima of these bacteria (Perombelon and Kelman 1980). Nonetheless, according to the herein reported study, coinfections with diverse SRP tend to be more common than it was previously thought.
The appropriate provision of plants with micro and macro elements is essential for their growth, development and resistance to environmental factors including phytopathogens. Also the impact of natural microflora, which is influenced to a higher extent by cropping and management practices than the soil type, was pointed as important for the suppression of plant pathogens (Huber and Watson 1970). Therefore, we studied the fertilizing methods applied on potato fields in relation to the frequency of SRP isolations. Unfortunately, no obvious patterns were found. However, it seems interesting that over 50% of plant samples yielding P. atrosepticum each year were subjected to organic fertilizers or NPK supplemented with organics, which mostly included animal manures. It is in agreement with the fact that P. atrosepticum can efficiently utilize nutrients available in swine, cattle orsheep manures (Sledz et al. 2017).
In conclusion, the herein described study devoted to the monitoring of SRP on seed potato fields in Poland in 2013 and 2014 revealed that P. atrosepticum, P. carotovorum and P. parmentieri were widespread in our country and outnumbered the D. dianthicola and D. solani isolates. Basing on the results of our former research, we state that since its appearance in 2005, Dickeya spp. never played a dominating role in the SRP population in Poland. During both years of study, thelargest number of SRP was isolated in July and it turned out to be the only month in which the presence of bacteria from all the analysed species was confirmed on seed potato fields in Poland. During the performed studies we confirmed co-infections of potato plants by bacteria from 2 or 3 different species. The most common coinfections were these of P. parmentieri and P. carotovorum. Besides, our data indicated that SRP strains were isolated from majority of the tested potato cultivars. The conducted studies on the environmental sources and occurrence patterns of soft rot pathogens might contribute to more efficient evaluation of the risks associated with infections caused by distinct SRP species.
References
Adeolu, M., Alnajar, S., Naushad, S., & Gupta, R. S. (2016). Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: proposal for Enterobacterales Ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. International Journal of Systematic and Evolutionary Microbiology, 66(12), 5575–5599. https://doi.org/10.1099/ijsem.0.001485.
Barrett, L. G., Kniskern, J. M., Bodenhausen, N., Zhang, W., & Bergelson, J. (2009). Continua of specificity and virulence in plant host–pathogen interactions: Causes and consequences. New Phytologist, 183(3), 513–529. https://doi.org/10.1111/j.1469-8137.2009.02927.x.
Cahill, G., Fraser, K., Kowalewska, M., Kenyon, D., & Saddler, G. (2010). Recent findings from the Dickeya survey and monitoring programme. In The Dundee Conference. Crop Protection in Northern Britain 2010 (p. 171–176.). Dundee, United Kingdom: The Association for Crop Protection in Northern Britain.
Cappaert, M. R., Powelson, M. L., Franc, G. D., & Harrison, M. D. (1988). Irrigation water as a source of inoculum of soft rot Erwinias for aerial stem rot of potatoes. Phytopathology, 78(12), 1668–1672. https://doi.org/10.1094/Phyto-78-1668.
Charkowski, A. O. (2018). The changing face of bacterial soft-rot diseases. Annual Review of Phytopathology, 56(1), 269–288. https://doi.org/10.1146/annurev-phyto-080417-045906.
De Boer, S. H., Li, X., & Ward, L. J. (2012). Pectobacterium spp. associated with bacterial stem rot syndrome of potato in Canada. Phytopathology, 102(10), 937–947. https://doi.org/10.1094/PHYTO-04-12-0083-R.
de Haan, E. G., Dekker-Nooren, T. C. E. M., van den Bovenkamp, G. W., Speksnijder, A. G. C. L., van der Zouwen, P. S., & van der Wolf, J. M. (2008). Pectobacterium carotovorum subsp. carotovorum can cause potato blackleg in temperate climates. European Journal of Plant Pathology, 122(4), 561–569. https://doi.org/10.1007/s10658-008-9325-y.
Dees, M. W., Lebecka, R., Perminow, J. I. S., Czajkowski, R., Grupa, A., Motyka, A., Zoledowska, S., Śliwka, J., Lojkowska, E., & Brurberg, M. B. (2017). Characterization of Dickeya and Pectobacterium strains obtained from diseased potato plants in different climatic conditions of Norway and Poland. European Journal of Plant Pathology, 148(4), 839–851. https://doi.org/10.1007/s10658-016-1140-2.
Degefu, Y., Potrykus, M., Golanowska, M., Virtanen, E., & Lojkowska, E. (2013). A new clade of Dickeya spp. plays a major role in potato blackleg outbreaks in North Finland. Annals of Applied Biology, 162(2), 231–241. https://doi.org/10.1111/aab.12020.
Fikowicz-Krosko, J., Wszalek-Rozek, K., Smolarska, A., & Czajkowski, R. (2017). First report on isolation of soft rot Pectobacterium carotovorum subsp. carotovorum from symptomless bittersweet nightshade occuring in rural area in Poland. Journal of Plant Pathology, 99(1), 294. https://doi.org/10.4454/jpp.v99i1.3772.
Frechon, D., Exbrayat, P., Helias, V., Hyman, L. J., Jouan, B., Llop, P., Lopez, M. M., Payet, N., Pérombelon, M. C. M., Toth, I. K., van Beckhoven, J. R. C. M., van der Wolf, J. M., & Bertheau, Y. (1998). Evaluation of a PCR kit for the detection of Erwinia carotovora subsp. atroseptica on potato tubers. Potato Research, 41(2), 163–173. https://doi.org/10.1007/BF02358439.
Gardan, L., Gouy, C., Christen, R., & Samson, R. (2003). Elevation of three subspecies of Pectobacterium carotovorum to species level: Pectobacterium atrosepticum sp. nov., Pectobacterium betavasculorum sp. nov. and Pectobacterium wasabiae sp. nov. International Journal of Systematic and Evolutionary Microbiology, 53, 381–391. https://doi.org/10.1099/ijs.0.02423-0.
Ge, T. L., Jiang, H. H., Hao, J. J., & Johnson, S. B. (2018). First report of Pectobacterium parmentieri causing bacterial soft rot and blackleg on potato in Maine. Plant Disease, 102(2), 437. https://doi.org/10.1094/PDIS-05-17-0659-PDN.
Golanowska, M., Kielar, J., & Lojkowska, E. (2017). The effect of temperature on the phenotypic features and the maceration ability of Dickeya solani strains isolated in Finland, Israel and Poland. European Journal of Plant Pathology, 147(4), 803–817. https://doi.org/10.1007/s10658-016-1044-1.
Golanowska, M., Potrykus, M., Motyka-Pomagruk, A., Kabza, M., Bacci, G., Galardini, M., Bazzicalupo, M., Makalowska, I., Smalla, K., Mengoni, A., Hugouvieux-Cotte-Pattat, N., & Lojkowska, E. (2018). Comparison of highly and weakly virulent Dickeya solani strains, with a view on the pangenome and panregulon of this species. Frontiers in Microbiology, 9, 1940. https://doi.org/10.3389/fmicb.2018.01940.
Golkhandan, E., Kamaruzaman, S., Sariah, M., Zainal Abidin, M. A., & Nasehi, A. (2013). Characterization of Malaysian Pectobacterium spp. from vegetables using biochemical, molecular and phylogenetic methods. European Journal of Plant Pathology, 137(3), 431–443. https://doi.org/10.1007/s10658-013-0270-z.
Gross, D. C., Powelson, M. L., Regner, K. M., & Radamaker, G. (1991). A bacteriophage-typing system for surveying the diversity and distribution of strains of Erwinia carotovora in potato fields. Phytopathology, 81(2), 220–226. https://doi.org/10.1094/phyto-81-220.
Helias, V. (2006). Potato blackleg in France: Incidence of causal Erwinia species and field symptoms expression, In 1st International Erwinia Workshop (p. 15). Scotland: Dundee.
Hélias, V., Hamon, P., Huchet, E., van der Wolf, J. M., & Andrivon, D. (2012). Two new effective semiselective crystal violet pectate media for isolation of Pectobacterium and Dickeya. Plant Pathology, 61(2), 339–345. https://doi.org/10.1111/j.1365-3059.2011.02508.x.
Huber, D. M., & Watson, R. D. (1970). Effect of organic amendment on soil-borne plant pathogens. Phytopathology, 60(1), 22–26.
Hugouvieux-Cotte-Pattat, N., Condemine, G., Nasser, W., & Reverchon, S. (1996). Regulation of pectinolysis in Erwinia chrysanthemi. Annual Reviews in Microbiology, 50(1), 213–257. https://doi.org/10.1146/annurev.micro.50.1.213.
Kang, H. W., Kwon, S. W., & Go, S. J. (2003). PCR-based specific and sensitive detection of Pectobacterium carotovorum ssp. carotovorum by primers generated from a URP-PCR fingerprinting-derived polymorphic band. Plant Pathology, 52(2), 127–133. https://doi.org/10.1046/j.1365-3059.2003.00822.x.
Kapsa, J., & Gawinska-Urbanowicz, H. (2013). Disease incidence on potato in Poland in the years 2009–2012. Progress in Plant Protection, 53(3), 545–551. https://doi.org/10.14199/ppp-2013-061.
Khayi, S., Cigna, J., Chong, T. M., Quêtu-Laurent, A., Chan, K. G., Hélias, V., & Faure, D. (2016). Transfer of the potato plant isolates of Pectobacterium wasabiae to Pectobacterium parmentieri sp. nov. International Journal of Systematic and Evolutionary Microbiology, 66(12), 5379–5383. https://doi.org/10.1099/ijsem.0.001524.
Kim, H. S., Ma, B., Perna, N. T., & Charkowski, A. O. (2009). Phylogeny and virulence of naturally occurring type III secretion system-deficient Pectobacterium strains. Applied and Environmental Microbiology, 75(13), 4539–4549. https://doi.org/10.1128/AEM.01336-08.
Laurila, J., Ahola, V., Lehtinen, A., Joutsjoki, T., Hannukkala, A., Rahkonen, A., & Pirhonen, M. (2008). Characterization of Dickeya strains isolated from potato and river water samples in Finland. European Journal of Plant Pathology, 122(2), 213–225. https://doi.org/10.1007/s10658-008-9274-5.
Ma, B., Hibbing, M. E., Kim, H. S., Reedy, R. M., Yedidia, I., Breuer, J., Breuer, J., Glasner, J. D., Perna, N. T., Kelman, A., & Charkowski, A. O. (2007). Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathology, 97(9), 1150–1163. https://doi.org/10.1094/PHYTO-97-9-1150.
Mansfield, J., Genin, S., Magori, S., Citovsky, V., Sriariyanum, M., Ronald, P., et al. (2012). Top 10 plant pathogenic bacteria in molecular plant pathology. Molecular Plant Pathology, 13(6), 614–629. https://doi.org/10.1111/j.1364-3703.2012.00804.x.
Motyka, A., Zoledowska, S., Sledz, W., & Lojkowska, E. (2017). Molecular methods as tools to control plant diseases caused by Dickeya and Pectobacterium spp: A minireview. New Biotechnology, 39, 181–189. https://doi.org/10.1016/J.NBT.2017.08.010.
Motyka-Pomagruk, A., Zoledowska, S., Misztak, A. E., & Sledz, W. (2020). Mengoni. A. & Lojkowska E. Comparative genomics and pangenome-oriented studies reveal high homogeneity of the agronomically relevant enterobacterial plant pathogen Dickeya solani. BMC Genomics, 21, 449. https://doi.org/10.1186/s12864-020-06863-w.
Nabhan, S., Wydra, K., Linde, M., & Debener, T. (2012). The use of two complementary DNA assays, AFLP and MLSA, for epidemic and phylogenetic studies of pectolytic enterobacterial strains with focus on the heterogeneous species Pectobacterium carotovorum. Plant Pathology, 61(3), 498–508. https://doi.org/10.1111/j.1365-3059.2011.02546.x.
Naumann, K., & Zielke, R. (1977). Latent infection of potato tubers with the bacterial soft rot pathogen Erwinia carotovora var. atroseptica, and its importance to low-loss potato storage. Nachrichtenblatt fu den Pflanzenschutz in der DDR, 31(1), 1–3.
Nowacki, W. (2012). Charakterystyka Krajowego Rejestru Odmian Ziemniaka. Jadwisin, Poland: Wydanie XV.
Nunes Leite, L., de Haan, E. G., Krijger, M., Kastelein, P., van der Zouwen, P. S., van den Bovenkamp, G. W., Tebaldi, N. D., & van der Wolf, J. M. (2014). First report of potato blackleg caused by Pectobacterium carotovorum subsp. brasiliensis in the Netherlands. New Disease Reports, 29, 24. https://doi.org/10.5197/j.2044-0588.2014.029.024.
Nykyri, J., Niemi, O., Koskinen, P., Nokso-Koivisto, J., Pasanen, M., Broberg, M., Plyusnin, I., Törönen, P., Holm, L., Pirhonen, M., & Palva, E. T. (2012). Revised phylogeny and novel horizontally acquired virulence determinants of the model soft rot phytopathogen Pectobacterium wasabiae SCC3193. PLoS Pathogens, 8(11), e1003013. https://doi.org/10.1371/journal.ppat.1003013.
Nykyri, J., Fang, X., Dorati, F., Bakr, R., Pasanen, M., Niemi, O., Palva, E. T., Jackson, R. W., & Pirhonen, M. (2014). Evidence that nematodes may vector the soft rot-causing enterobacterial phytopathogens. Plant Pathology, 63(4), 747–757. https://doi.org/10.1111/ppa.12159.
Olsson, K. (1985). Detection of Erwinia spp. in some Swedish streams. In Report of the international conference on potato blackleg disease (pp. 45–46). Oxford, United Kingdom: Oxford University Press.
Ozturk, M., Aksoy, H. M., Ozturk, S., Potrykus, M., & Lojkowska, E. (2016). First report of potato blackleg and soft rot caused by Pectobacterium wasabiae in Turkey. New Disease Reports, 34, 17. https://doi.org/10.5197/j.2044-0588.2016.034.017.
Ozturk, M., Aksoy, H. M., Potrykus, M., & Lojkowska, E. (2018). Genotypic and phenotypic variability of Pectobacterium strains causing blackleg and soft rot on potato in Turkey. European Journal of Plant Pathology, 152(1), 143–155. https://doi.org/10.1007/s10658-018-1459-y.
Palacio-Bielsa, A., Cambra, M. A., & Lopez, M. M. (2006). Characterisation of potato isolates of Dickeya chrysanthemi in Spain by a microtitre system for biovar determination. Annals of Applied Biology, 148(2), 157–164. https://doi.org/10.1111/j.1744-7348.2006.00045.x.
Pasanen, M., Laurila, J., Brader, G., Palva, E. T., Ahola, V., van der Wolf, J., Hannukkala, A., & Pirhonen, M. (2013). Characterisation of Pectobacterium wasabiae and Pectobacterium carotovorum subsp. carotovorum isolates from diseased potato plants in Finland. Annals of Applied Biology, 163(3), 403–419. https://doi.org/10.1111/aab.12076.
Perombelon, M. C. M. (2002). Potato diseases caused by soft rot erwinias: An overview of pathogenesis. Plant Pathology, 51(1), 1–12. https://doi.org/10.1046/j.0032-0862.2001.
Perombelon, M. C. M., & Kelman, A. (1980). Ecology of the soft rot Erwinias. Annual Review of Phytopathology, 18(1), 361–387. https://doi.org/10.1146/annurev.py.18.090180.002045.
Potrykus, M., Sledz, W., Golanowska, M., Slawiak, M., Binek, A., Motyka, A., Zoledowska, S., Czajkowski, R., & Lojkowska, E. (2014). Simultaneous detection of major blackleg and soft rot bacterial pathogens in potato by multiplex polymerase chain reaction. Annals of Applied Biology, 165(3), 474–487. https://doi.org/10.1111/aab.12156.
Potrykus, M., Golanowska, M., Sledz, W., Zoledowska, S., Motyka, A., Kolodziejska, A., Butrymowicz, J., & Lojkowska, E. (2016). Biodiversity of Dickeya spp. isolated from potato plants and water sources in temperate climate. Plant Disease, 100(2), 408–417. https://doi.org/10.1094/PDIS-04-15-0439-RE.
Powelson, M. L. (1980). Seasonal incidence and cause of blackleg and a stem soft rot of potatoes in Oregon. American Potato Journal, 57(7), 301–306. https://doi.org/10.1007/BF02854024.
Rossmann, S., Dees, M. W., Perminow, J., Meadow, R., & Brurberg, M. B. (2018). Soft rot Enterobacteriaceae are carried by a large range of insect species in potato fields. Applied and Environmental Microbiology, 84(12), e00281–e00218. https://doi.org/10.1128/AEM.00281-18.
Samson, R., Legendre, J. B., Christen, R., Fischer-Le Saux, M., Achouak, W., & Gardan, L. (2005). Transfer of Pectobacterium chrysanthemi (Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen. nov. as Dickeya chrysanthemi comb. nov. and Dickeya paradisiaca comb. nov. and delineation of four novel species, Dickeya dadantii sp. nov., Dickeya dianthicola sp. nov., Dickeya dieffenbachiae sp. nov. and Dickeya zeae sp. nov. International journal of systematic and evolutionary microbiology, 55(Pt 4), 1415–27. https://doi.org/10.1099/ijs.0.02791-0
Slawiak, M., Łojkowska, E., & van der Wolf, J. M. (2009). First report of bacterial soft rot on potato caused by Dickeya sp. (syn. Erwinia chrysanthemi) in Poland. Plant Pathology, 58(4), 794–794. https://doi.org/10.1111/j.1365-3059.2009.02028.x.
Slawiak, M., van Doorn, R., Szemes, M., Speksnijder, A. G. C. L., Waleron, M., van der Wolf, J. M., et al. (2013). Multiplex detection and identification of bacterial pathogens causing potato blackleg and soft rot in Europe, using padlock probes. Annals of Applied Biology, 163(3), 378–393. https://doi.org/10.1111/aab.12075.
Sledz, W., Jafra, S., Waleron, M., & Lojkowska, E. (2000). Genetic diversity of Erwinia carotovora strains isolated from infected plants grown in Poland. EPPO Bulletin, 30(3–4), 403–407. https://doi.org/10.1111/j.1365-2338.2000.tb00919.x.
Sledz, W., Zoledowska, S., Motyka, A., Kadzinski, L., & Banecki, B. (2017). Growth of bacterial phytopathogens in animal manures. Acta Biochimica Polonica, 64(1), 151–159. https://doi.org/10.18388/abp.2016_1389.
Stypa, I. (2015). Charakterystyka odmian ziemniaka. Bonin, Poland: IHAR – PIB Zakład Nasiennictwa i Ochrony Ziemniaka Bonin.
Suárez, M. B., Feria, F. J., Martín-Robles, M. J., del Rey, F. J., & Palomo, J. L. (2017). Pectobacterium parmentieri causing soft rot on potato tubers in southern Europe. Plant Disease, 101(6), 1029. https://doi.org/10.1094/PDIS-01-17-0013-PDN.
Toth, I. K., van der Wolf, J. M., Saddler, G., Lojkowska, E., Helias, V., Pirhonen, M., et al. (2011). Dickeya species: An emerging problem for potato production in Europe. Plant Pathology, 60(3), 385–399. https://doi.org/10.1111/j.1365-3059.2011.02427.x.
Tsuyama, H., & Sakamoto, M. (1952). Studies on the soft-rot causing bacteria of vegetables inhabiting in the soil. Science Reports of the Research Institutes, 4, 81–95.
van Beckhoven, J., van Hoof, R., De Raaij, G., Bonants, P., & van der Wolf, J. M. (2001). Eindverslag survey naar serogroepen van Erwinia carotovora subsp. atroseptica en E. chrysanthemi in aardappel in Nederland. In Intern rapport Plant Research International. Wageningen, The Netherlands: Plant Research International.
van der Wolf, J. M., & Kastelein, P. (2014). The role of haulm infections in the epidemiology of soft rot Enterobacteriaceae. In The 3rd International Erwinia Workshop on soft rot Enterobacteriaceae and related organisms (pp. 7, S1-K1).
van der Wolf, J. M., Nijhuis, E. H., Kowalewska, M. J., Saddler, G. S., Parkinson, N., Elphinstone, J. G., Pritchard, L., Toth, I. K., Lojkowska, E., Potrykus, M., Waleron, M., de Vos, P., Cleenwerck, I., Pirhonen, M., Garlant, L., Hélias, V., Pothier, J. F., Pflüger, V., Duffy, B., Tsror, L., & Manulis, S. (2014). Dickeya solani sp. nov., a pectinolytic plant-pathogenic bacterium isolated from potato (Solanum tuberosum). International Journal of Systematic and Evolutionary Microbiology, 64, 768–774. https://doi.org/10.1099/ijs.0.052944-0.
van der Wolf, J. M., de Haan, E. G., Kastelein, P., Krijger, M., de Haas, B. H., Velvis, H., Mendes, O., Kooman-Gersmann, M., & van der Zouwen, P. S. (2017). Virulence of Pectobacterium carotovorum subsp. brasiliense on potato compared with that of other Pectobacterium and Dickeya species under climatic conditions prevailing in the Netherlands. Plant Pathology, 66(4), 571–583. https://doi.org/10.1111/ppa.12600.
Waleron, M., Waleron, K., Podhajska, A. J., & Łojkowska, E. (2002). Genotyping of bacteria belonging to the former Erwinia genus by PCR-RFLP analysis of a recA gene fragment. Microbiology, 148(2), 583–595. https://doi.org/10.1099/00221287-148-2-583.
Waleron, M., Waleron, K., & Lojkowska, E. (2013). Occurrence of Pectobacterium wasabiae in potato field samples. European Journal of Plant Pathology, 137(1), 149–158. https://doi.org/10.1007/s10658-013-0227-2.
Yishay, M., Burdman, S., Valverde, A., Luzzatto, T., Ophir, R., & Yedidia, I. (2008). Differential pathogenicity and genetic diversity among Pectobacterium carotovorum ssp. carotovorum isolates from monocot and dicot hosts support early genomic divergence within this taxon. Environmental Microbiology, 10(10), 2746–2759. https://doi.org/10.1111/j.1462-2920.2008.01694.x.
Zarzecka, K. (2009). Potato as a global plant nutritional dietary and medicinal values. In Rozprawy Naukowe PWSZ im. Jana Pawła II w Białej Podlaskiej (Vol. 3, pp. 163–175). Biała Podlaska, Poland: Państwowa Szkoła Wyższa im. Papieża Jana Pawła II w Białej Podlaskiej.
Zoledowska, S., Motyka-Pomagruk, A., Sledz, W., Mengoni, A., & Lojkowska, E. (2018a). High genomic variability in the plant pathogenic bacterium Pectobacterium parmenieri deciphered from de novo assembled complete genomes. BMC Genomics, 19(1), 751. https://doi.org/10.1186/s12864-018-5140-9.
Zoledowska, S., Motyka, A., Zukowska, D., Sledz, W., & Lojkowska, E. (2018b). Population structure and biodiversity of Pectobacterium parmentieri isolated from potato fields in temperate climate. Plant Disease, 102(1), 154–164. https://doi.org/10.1094/PDIS-05-17-0761-RE.
Acknowledgements
This work was funded by National Science Centre in Poland (Narodowe Centrum Nauki) via projects: UMO-2014/14/M/NZ8/00501 attributed to EL and UMO-2016/21/N/NZ1/02783 attributed to AM-P. The authors are grateful to Dr. Malgorzata Golanowska and Dr. Marta Potrykus for assistance in isolation of several strains described in this research. The authors would like to thank the inspectors from The State Plant Health and Seed Inspection Service in Poland and the employees of other collaborating institutions for the provision of the soft rot- and blackleg-affected plants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Human and/or animals rights
Not applied.
Informed consent
Not applied.
Supplementary Information
ESM 1
(DOCX 672 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Motyka-Pomagruk, A., Zoledowska, S., Sledz, W. et al. The occurrence of bacteria from different species of Pectobacteriaceae on seed potato plantations in Poland. Eur J Plant Pathol 159, 309–325 (2021). https://doi.org/10.1007/s10658-020-02163-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-02163-x


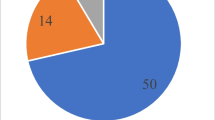







 – Dickeya spp. (Dsp),
– Dickeya spp. (Dsp),  – P. parmentieri (Ppa),
– P. parmentieri (Ppa),  – P. atrosepticum (Pba),
– P. atrosepticum (Pba),  – P. carotovorum (Pc)
– P. carotovorum (Pc)