Abstract
Pseudomonas syringae pv. actinidiae (Psa) has caused severe damage to kiwifruit plantations worldwide. It is still crucial to find compounds that are able to control the disease. In this work we developed a time and space-saving leaf disk assay using highly susceptible yellow-fleshed cultivars. The assay enabled 29 molecules/compounds to be screened in relation to their capacity to condition the development of necrosis on leaf disks by treating them before the inoculation. A necrosis index was thereby created. Fosetyl-aluminum, DL-β-Amino-n-butyric acid (BABA) and saccharin (BIT) reduced symptoms significantly, and Fosetyl-aluminum was the most effective. Maxim®, triclopyr and Methyl jasmonate enhanced the necrosis index significantly. S-Methyl 1,2,3-benzothiadiazole-7-carbothioate (BTH) also enhanced symptom severity, though there was no statistical support confirming this. Salicylic acid did not condition symptom development. In vitro investigations into the direct effect of the compounds on Psa highlighted that Fosetyl-aluminum and the polyamines, spermidine and cadaverine, inhibited growth and also had a biocidal effect in a dose-dependent fashion. Salicylic acid and spermine also strongly reduced bacterial growth. Long-term trials with potted plants in the greenhouse confirmed the protective effect of Fosetyl-aluminum and clearly showed that BTH enhances the susceptibility of kiwifruit to Psa. Fosetyl-aluminum significantly decreased symptom expression in kiwifruit plantations (cv. Hayward) subjected to natural Psa infection. Monitoring Psa growth dynamics in the leaf disks by time-point-based Real-Time PCR, showed that treatment with Fosetyl-aluminum restricts bacterial multiplication within the host plant.
Similar content being viewed by others
Introduction
Pseudomonas syringae pv. actinidiae (Psa) is the cause of bacterial canker of kiwifruit and is seriously damaging plantations in many countries worldwide (Scortichini et al. 2012; Vanneste 2017). To date, based on genomic features and the level of virulence, five populations – biovar 1, 2, 3, 5, 6 – have been described within the source population of Psa (Chapman et al. 2012; McCann et al. 2013; Ferrante and Scortichini 2015; Fujikawa and Sawada 2016; McCann et al. 2017; Fujikawa and Sawada 2019).
Psa-1 was first isolated in Japan in 1984 (Takikawa et al. 1989) and in Italy in 1992 (Scortichini 1994). Psa-2 was reported in Korea in 1994 (Koh et al. 1994). In 2008 a pandemic outbreak caused by Psa-3 appeared and spread for the first time in Italy (Balestra et al. 2008; Ferrante and Scortichini 2009) with reports in neighboring European countries, New Zealand, Asia and Chile occurring soon after (Vanneste 2017). The outbreak by Psa-3 led to unprecedented heavy damage of the plantations with consequent serious economic losses. The awareness of biovar identity was postponed from the first reports and came for the first time from Chapman et al. (2012). Biovars 5 and 6 were recorded in restricted areas in Japan in 2014 and 2015, respectively, and were considered endemic lineages (Fujikawa and Sawada 2016; Fujikawa and Sawada 2019).
China is considered the origin of the pandemic lineage of Psa-3, from which it spread worldwide by distinct transmission events (Mazzaglia et al. 2012; Butler et al. 2013; McCann et al. 2017). The main symptoms of the pandemic bacterial canker are: leaf spotting, twig wilting, flower necrosis, and cankers on the main trunk and leaders with sunken bark oozing out white or red exudates.
Field observations, subsequently confirmed by Koch postulate-based studies, strongly suggest that A. chinensis var. chinensis (yellow-fleshed) is more susceptible than A. chinensis var. deliciosa (green-fleshed) to the pandemic Psa-3, although even the latter can be severely affected (Ferrante and Scortichini 2009; Marcelletti et al. 2011; McCann et al. 2013).
With regard to control strategies, antibiotics are legal in Asia and New Zealand for controlling Psa (Cameron and Sarojini 2014), but not in Italy and the rest of the EU. Moreover, the insurgence of antibiotic-resistant Psa strains in Japan and Korea (Han et al. 2003) makes this strategy inadvisable.
A wide range of compounds and molecules have thus been tested. Among those with a direct antibacterial activity, copper protects from new infection events in autumn and winter during which fruit harvesting, leaf fall, pruning and sudden drop in temperature, create wounds through which the bacterium enters and colonizes the plant. Copper compounds are also useful during the vegetative season, if applied soon after adverse climate events and green pruning (Fratarcangeli et al. 2010; Quattrucci et al. 2010; Monchiero et al. 2015). However, in this period the treatments should be limited and maintained under specific threshold doses in order not to cause phytotoxicity (Fratarcangeli et al. 2010). Moreover copper is not systemic thus it is not helpful when the bacterium has systemically colonized the plant. Massive applications should also be avoided due to possible accumulation in the soil and to the fact that Psa can acquire copper resistance in field conditions (Colombi et al. 2017).
Chitosan-based compounds (e.g. Hendophyit PS) have also been found to decrease/delay bacterial canker progress (Scortichini 2014). The in vitro biocidal activity against Psa suggests that such compounds have a direct mode of action in field applications (Ferrante and Scortichini 2010; Scortichini 2014). In addition, Corsi et al. (2017) reported that chitosan elicits PR proteins in artificially infected kiwi plants suggesting that the activation of defence responses might also play a role.
Several natural compounds and essential oils have been tested for their antibacterial activity against Psa, some of which showed an in vitro efficacy, though this is still to be verified in in planta trials (Vavala et al. 2016; Pucci et al. 2018; Simonetti et al. 2019).
BTH or acibenzolar-S-methyl (IUPAC name: S-Methyl 1,2,3-benzothiadiazole-7-carbothioate; commercial product: Bion® or Actigard®, Syngenta, Basel, Switzerland) is a systemic molecule and the functional analogue of the stress hormone salicylic acid (SA). Like SA, BTH has been shown to work as a resistance inducer in many plant pathosystems (Faize and Faize 2018). In kiwifruit, field applications of BTH have been repeatedly reported to confer some protection, though not decisive, against bacterial canker symptoms (Valente et al. 2014; Tosi et al. 2014; Pizzinat et al. 2014; Antoniacci et al. 2014), along with phytotoxic effects (Pizzinat et al. 2014; Monchiero et al. 2015; De Jong et al. 2019). In 2017, BTH was authorized in Italy with an emergency procedure for use on kiwifruit for the control of bacterial canker (Ministry of Health, executive decree of 10 April 2017).
The main objective of this work was the identification of compounds able to control bacterial canker or with a potential for control. We thus aimed to: (i) develop a leaf disk-based treatment-and-inoculation assay in order to rapidly screen 29 compounds through a necrosis index; (ii) verify in vitro the direct inhibitory effects of the compounds on Psa; and (iii) confirm the leaf disk assay response of some selected compounds by greenhouse and field trials.
Materials and methods
Test organisms and chemical/natural compounds
Leaves of 2–4 year-old plants of A. chinensis var. chinensis cv. Belen and A. chinensis var. deliciosa cv. Hayward were used for the leaf disk assay.
Greenhouse plant assay was performed with six-month-old plants of A. chinensis var. chinensis cv. Soreli propagated in vitro from meristem tips of Psa-free mother plants (Simeoni Vivai Actinidia, Sacile, Pordenone, IT). The pot substrate was Radicom (Vigorplant, Lodi, Italy), containing a mixture of peat moss, black peat, marsh peat, and vegetable compost. Humus is present in this substrate in the form of humic and fulvic acids with a water pH of 6–6.5. The tests were conducted in a quarantine greenhouse at 20 °C and subjected to the natural photoperiod.
The field trials were conducted in two plantations of A. chinensis cv. Hayward.
Compounds tested in vitro were natural and synthetic plant hormones and molecules, silicon-based inorganic compounds and dehydrated herboristic plant powders (Table 1).
Psa strain CREA-DC 1625 (Psa-3) was used in all the assays. The bacterium was routinely grown on NDA (nutrient agar containing 0.25% D-glucose – Oxoid, Thermo Fisher Scientific, Waltham, Massachusetts USA), at 25–27 °C for 48 h and long-termed stored as lyophilized culture at 4 °C.
Bacterial growth test in compound-supplemented media to assess the inhibitive effect on Psa
Psa bacterial growth and viability were tested in liquid (minimum inhibitory concentration, MIC) and agarized media supplemented with the compounds under study.
A liquid starter culture was prepared by picking a single colony grown on NDA in 4–5 ml of nutrient broth supplemented with 5% sucrose (NSB, Oxoid) and incubated at 25 °C over-night at 170 rpm. Bacterial concentration was adjusted to 10^8 CFU/ml (Colony Forming Units) based on spectrophotometer readings at an absorbance of 660 nm. The starter culture was then diluted (1:150) and divided into 15 ml aliquots, which were supplemented with compound doses in order to obtain seven different concentrations (for chemically standardized compounds: 0.02, 0.2, 0.4, 1, 2, 4, 8 mM).
Five culture-replicates per treatment/concentration were used. Untreated Psa cultures were used as controls (control 1, C1). When a diluent was needed to dissolve a compound, then C1 was supplemented with it. Negative controls were NSB supplemented with each compound/diluent at the highest concentration used (control 2, C2). The cultures were incubated overnight at 25 °C, 170 rpm. The optical density of all treatments was measured at 660 nm at about 20–22 h, when untreated Psa cultures (C1) reached approximately 1.0. Replicates for each treatment/concentration were then pooled, ten-fold serially diluted and 100 μl aliquots were plated on NDA, three plates for each dilution. After incubation, the presence of bacterial growth was recorded.
Not all the compounds of those that were tested in the leaf disk assay were tested in Psa cultures because of the high turbidity of the liquid medium after resuspending (the herboristic and silicon-based compounds and BTH). The resuspension of Fosetyl-aluminum and isotianil also caused a dose-dependent turbidity, which however did not impede the spectrophotometrical detection of the bacterial turbidity by using NSB supplemented with the product as a blank.
The activity of Fosetyl-aluminum was further investigated with regard to: i) the ability to modify pH; ii) uncoupling the specific effect on Psa due to pH lowering from other effects inherent in its molecular structure.
i) pH was measured in both water and NSB which had been neutralized and then supplemented with Fosetyl-aluminum at 0.2 mM and the concentration series suggested for field treatments in the “how-to-use” label of the commercial compound i.e.: 1.1, 5.6, 9, and 22.6 mM. Measurements were performed on three sample-replicates for each medium and concentration. The experiments were repeated three times.
ii) NSB medium was supplemented with Fosetyl-aluminum at 1.1 and 5.6 mM. The pH of half the medium, for each concentration, was neutralized with 2 N NaOH. Then, Psa cultures on neutralized and non-neutralized media were performed and compared. Cultures were ten-fold serially diluted and plated on agarized medium as described above.
All the compounds under study, details on their dissolution or resuspension, and the tested concentrations are reported in Table 1 and Online Resource 1.
Leaf disk assay to screen the effects of the compounds on Psa
To screen the potential of a high number of compounds to influence Psa symptom development, we performed a leaf disk assay according to the following procedure.
Specifically, fully expanded leaves were collected from June to September, from nursery-grown equal-in-age trees. Leaves with the same degree of tenderness were collected from the fifth-eighth node of actively growing twigs.
Soon after leaf detachment, 2.3 cm diameter disks were punched out from the leaf blade, randomized, and immersed for 1 h at 23 °C under gentle agitation in the compound solution which contained 0.005% (v/v) Silwet L-77 as a wetting agent. The treatment was repeated after 24 h. In the time-span between the two treatments the leaf disks were incubated in water-agar plates (WA) (Oxoid) – five disks for each plate – at 20 °C in a growth chamber (CFT 1200, Piardi, Brescia ITA) with a photoperiod of 14/15 h-day and 9/10 h-night.
Soon after the second treatment, the disks were washed in water and then were challenge inoculated by immersing them into a 10^8 CFU/mL Psa suspension containing 0.01% (v/v) Silwet L-77, for 1 h at 23 °C under gentle agitation. Leaf disks were then incubated in WA plates at 15 °C in the growth chamber, as described above. Compound and bacterial incubation were always conducted in 90 mm Petri plates, and leaf disks were always immersed with the adaxial face uppermost, whereas incubation in WA plates was with the abaxial surface uppermost. In each experiment, each treatment consisted of 10 disk replicates placed into two separate Petri plates.
The positive control was treated twice with Silwet L-77, 24 h apart, to replace compound treatment, and was then challenge inoculated (non-treated Psa-control, NT-Psa).
Non-inoculated controls which had been treated and not treated with the compound (treated control, T and non-treated control NT), were always included in the experiments in order to evaluate any compound and senescence-linked necrosis potentially due to Silwet L-77, the dissolvents, all the compounds under study at the tested concentrations, and the wounds caused by the leaf blade cut (i.e. the phytotoxicity and wounding controls). These controls were prepared according to the above-described protocol. They were not inoculated with Psa and the unperformed steps were substituted with equivalent Silwet L-77-water baths.
The concentrations for using the compounds were selected from the literature on their use in plants for disease control or growth regulation as well as from the how-to-use labels of commercial products regarding field applications. Multiple concentrations were tested for BABA, BTH, Fosetyl-aluminum, Maxim®, and triclopyr (Table 1). For each compound, the experiment was repeated from four to 10 times. Symptom severity of leaf disks was recorded by three operators, by annotating in decimals (range 0–1) the extent of the necrotized leaf area. Annotations were then transformed according to a rating scale, in which increasing scores were attributed each to different ranges of necrotized leaf area: 0 (attributed to disks free of any visible necrosis), 1 (necrotic lesions from 0.01–0.12), 2 (0.13–0.25), 3 (0.26–0.37), 4 (0.38–0.5), 5 (0.51–0.62), 6 (0.63–0.81), and 7 (0.82–1). Disease severity was recorded twice, at 7–10 and 13–16 days post inoculation. A disease/necrosis index was then calculated, for each treatment/experiment, using the McKinney formula: [∑ (cl × v)] × 100/(N × CL), where: cl = score of the rating scale; v = number of disks attributed to cl; N = observed disks for each treatment, i.e. 10; CL = the highest value of the rating scale (7, in this work) (McKinney 1923).
A. chinensis cv. Belen was used to develop the leaf disk assay and screen the compounds. Some compounds for which necrosis index resulted significantly lower than NT-Psa control, were also tested on A. chinensis cv. Hayward for further confirmation.
Monitoring Psa with real-time PCR in Fosetyl-aluminum and water-treated leaf disks
To investigate the effects of Fosetyl-aluminum on the multiplication dynamics of Psa within leaf disks we used Real-Time PCR (Gallelli et al. 2014) for bacterium quantification in time point-based experiments.
Leaf disks were collected from two-year-old plants of A. chinensis cv. Soreli, treated with Fosetyl-aluminum at 9 mM (Swan) and water, and inoculated as described in the section regarding leaf disk assay. Each treatment/time point consisted in 15 disks equally distributed in three plates, which were considered as three biological replicates. We planned six time points: 0, 4, 8, 24, 72, 144 h. This experiment was repeated twice. When each time point had expired, the disks were washed three times in 0.1% and 0.05% Tween-20 and autoclaved distilled water respectively, for 8 min each time. After a rapid draining, the five disks of each plate were frozen in liquid nitrogen and stored together at −80 °C. DNA extraction was performed using DNeasy Plant Maxi Kit (Qiagen, Hilden, Germany) and quantified using a NanoDrop™ 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts).
The relative quantification method of Livak and Schmittgen (2001) was applied to Real-Time PCR Ct values obtained from DNA amplification of Psa (Gallelli et al. 2014) and β-tubulin (TUB) of kiwifruit. This latter was the internal control (Petriccione et al. 2015) and thus used to normalize Psa DNA quantity. SsoFast™ EvaGreen® Supermix and the CFX96™ thermocycler (Bio-Rad, Hercules, CA, USA) were used for PCR amplifications. Real-time PCR were performed as published, except for TUB amplification where the annealing/extension phase was performed at 62 °C. The efficiency of 100% for both Real-Time PCR was assessed by inferring standard curves (data not shown). A total of 25 ng of each DNA sample was used as a template in the PCR reactions.
Quantification analysis and normalization was according to Livak and Schmittgen (2001) which enabled to infer the Normalized relative Pathogen Quantity (NoPQ).
Greenhouse plant assay with Fosetyl-aluminum and BTH
A whole-plant assay was performed on A. chinensis cv. Soreli about 25 cm in height. The aim was to evaluate the effect of 9 mM Fosetyl-aluminum and 0.475 mM BTH on the inoculation with Psa. We used Helios WG Top (Sipcam) as the source of Fosetyl-aluminum (80% of the active principle), and Bion® as the source of BTH (50% of the active principle).
We performed three trials: A, B1 and B2. Trial A and the two B trials differed in terms of treatment calendar and method of inoculation. All treatments were performed on the soil with aqueous suspensions. An exception was the treatment in autumn (November) – when the foliage was still green – which consisted of spraying the compound suspension supplemented with 0.05% Silwet L-77 on both the adaxial and abaxial faces of the leaves. The treatment in March was a few days before sprouting. Details of treatment calendar and how the plants were grown are reported in Table 2. The temperature-conditioning system was kept off in autumn and winter. Psa inoculation was performed following two methods. i) Trial A: a bacterial suspension at 10^8 CFU/ml and 0.01% Silwet L-77 was sprayed with a dispenser-pump to the abaxial face of three fully-expanded leaves for each plant, located in the medium-apical trait of the stem. ii) Trial B: the bacterial suspension was syringe-injected at the two first nodes of the stem, near the leaf insertion, 100 μl for each injection point. Trial B was repeated twice: B1 and B2.
The experimental groups of plants inoculated with Psa were plants treated with the two compounds separately and, as a control, untreated plants (12 plants for each group). Similarly, the non-inoculated controls were treated and untreated plants (eight plants for each group).
Symptom severity was rated with different scores for the different classes of symptoms (Online Resource 2). The severity for each plant depended on the occurrence of a single symptom class, represented by a single score, or a combination of symptom classes, evaluated as a score sum. The class combinations which included the highest-valued class (death) were valued as this latter (Online Resource 2). A disease index was then calculated using the McKinney formula (McKinney, 1923).
Petiole and leaf samples were collected to assess the presence of Psa in the plants still surviving at the end of the trials. Isolation on NSA medium and Real-Time PCR were used for detection (Gallelli et al. 2011; Gallelli et al. 2014; OEPP/EPPO 2014).
Field trial to test Fosetyl-aluminum efficacy against Psa
To confirm the effect of Fosetyl-aluminum in field conditions, i.e. in commercial kiwifruit orchards, 2–4-month trials were performed to assess the capacity of the compound to influence the evolution of natural Psa symptoms. The trials were performed in 2015 in two kiwifruit orchards located in Borgo Montello (Latina, Latium, Italy) which we refer to as field 1 and field 2. The trees were A. chinensis cv. Hayward, spaced at 5 × 4 m., and trained on a pergola. In fields 1 and 2, the trees were 3 and 10 years old, respectively. Three adjacent experimental groups were established in each orchard, each group was composed of 90 trees and given a specific treatment. Within each group, an experimental plot of 30 trees was designed and specifically addressed to disease recording. Thus, each of the three 30-tree experimental plots was surrounded by a buffer zone of trees subjected to the same treatments. This buffer zone was included to minimize any edge effects.
Rainfall and average maximum and minimum temperature were obtained from the meteorological station located in the area (Cisterna di Borgo Carso – Servizio integrato agrometeorologico, Arsial, Latium). We tested Fosetyl-aluminum (Helios WG Top) at 5 and 10 g/l water, corresponding to 11.3 and 22.6 mM active principle, compared with an untreated experimental plot. The treatment consisted in supplementing the soil around the tree collar with two liters of compound suspension. Treatments were performed three times in spring 2015 – 10 April, 24 April and 13 May. Disease incidence was estimated by annotating the number of leaves per tree that were affected by necrotic angular spots typical of Psa infection. Estimating the number of leaves per tree (through counting a quarter of the whole canopy) revealed the percentage of the canopy with leaf symptoms. The number of canker-affected branches per tree and the number of dead trees with typical Psa ooze were also recorded. Symptoms were recorded at the beginning of the trial, on 9 April, then on 29 May and 8 July.
Branch and leaf samples were collected to confirm the presence of Psa in the orchard at the start of the trial, and to confirm the association between recorded symptoms and Psa. Detection methods were isolation on NSA medium and Real-Time PCR (Gallelli et al. 2011; Gallelli et al. 2014; OEPP/EPPO 2014).
Statistical analysis
A statistical analysis was performed to assess differences between the experimental groups.
A one-way fully random analysis of variance (ANOVA) was carried out, using the Student-Newman-Keuls test as a multiple testing procedure (post hoc analysis). When a data group violated ANOVA assumptions, we used Welch’s test coupled with Tukey as post hoc (normal distribution, heteroscedasticity), Kruskal-Wallis coupled with Mann-Whitney Bonferroni corrected (non-normal distribution, homoscedasticity), and Friedman coupled with Wilcoxon (non-normal distribution, heteroscedasticity). STATGRAPHICS® Centurion XVI and PAST v. 2.17 were used for the analyses. With regard to the leaf disk assay and the trials on potted plants, values of disease/necrosis index were the input of the analyses.
Results
The inhibitive effect of the compounds on Psa in in vitro growth tests
We investigated the direct effect of each compound on Psa, by measuring bacterial growth in liquid cultures, and then checking the viability by plating on agarized media.
For triclopyr, kinetin, probenazole (PBZ) and isotianil, we analyzed only the lowest concentrations of the series, as testing higher concentrations entailed the use of diluents (aceton and NaOH, see Online Resource 1) in a sufficient quantity to inhibit the bacterial growth and thus mask the real effect of the compound under study.
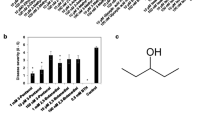
Several compounds – 1-aminocyclopropane-1-carboxilic acid (ACC), gibberellic acid (GA), MeJA, 1-naphthaleneacetic acid (NAA), gentisic acid, isotianil, saccharin (BIT), and ornithine – slightly inhibited the bacterial growth at all or nearly all tested concentrations, compared with the control (p < 0.01). No significant differences were detected with respect to BABA, kinetin (KI), triclopyr and putrescine. Strong growth inhibition was obtained with Fosetyl-aluminum, indole-3-acetic acid (IAA), SA, PBZ, cadaverine, spermidine and spermine (p < 0.01). Overall, growth inhibition was dose dependent. No bacterial growth was recorded after plating liquid cultures with Fosetyl-aluminum (at 8 mM), cadaverine (starting with 4 mM) and spermidine (starting with 2 mM) on the agarized medium, thus highlighting a bactericidal effect. See Fig. 1 and Online Resource 3.
Optical density (OD) of Pseudomonas syringae pv. actinidiae (Psa) after growing in NSB supplemented with compounds/molecules at different concentrations. These were also used in the leaf disk assay. All measurements were made with a spectrophotometer at 660 nm after 20–22 h incubation, when non-compound-supplemented Psa cultures (called Control 1, C1) reached approximately the value of 1.0. Control 2 (C2) is the absorbance of non-inoculated NSB. Different letters indicate significant differences between groups (p < 0.01 for Fosetyl-aluminum, salicylic acid and spermidine and p < 0.03 for BABA). The vertical bars indicate standard deviations of the means. Asterisks indicate the bactericidal concentrations. Here we show four representative growth trends. In Online Resource 3 we depict growth of Psa on NSB supplemented with 19 compounds/molecules
Further investigations were performed on Fosetyl-aluminum. The pH measurements in water and NSA supplemented with Fosetyl-aluminum proved that it is an acidifying salt, at least in an amphoteric environment. Specifically, pH decreased significantly and proportionally to the increase in compound concentration. In water, the decrease in pH was significantly more pronounced than in NSA (Fig. 2). (p < 0.01).
Acidification capacity of Fosetyl-aluminum at the concentrations suggested for field treatments on the product label. pH values marked with different letters are significantly different (p < 0.01). The vertical bars indicate standard deviations of the means. Each concentration was tested in three independent samples and the experiment was repeated three times
Growth analysis of Psa in neutralized and non-neutralized liquid medium supplemented with Fosetyl-aluminum revealed that 1.12 mM Fosetyl-aluminum dramatically decreased the bacterial growth with 1.07 × 10^2 CFU/ml (SE ± 4.5 × 10^1) for non-neutralized cultures and 1.15 × 10^4 CFU/ml (SE ± 2.82 × 10^3) for neutralized cultures, while the control culture (not supplemented with Fosetyl-aluminum) yielded thick plaques (p < 0.0024). At 5.56 mM compound both neutralized and non-neutralized cultures were bactericidal as no bacterial growth was observed after plating on the agarized medium at all serial dilutions.
Screening of compounds by leaf disk assay for their capacity to decrease/increase Psa symptoms
The assay enabled a clear and fast symptom expression after bacterial inoculation of leaf disks from both Belen and Hayward. Symptoms started from the fifth to the eighth day after inoculation. Necrotic lesions progressively increased their size and their number till coalescence. Within 14–20 days, spreading necrosis affected the entire leaf disk surface (Fig. 3).
The untreated and non-inoculated controls (NT) never showed wound and time-linked senescence over the lifetime of the experiments and appeared green and healthy-looking up to 1 month, after which some chlorosis appeared. The treated and non-inoculated controls (T) did not show any necrotic lesions related to compound phytotoxicity (Online Resource 4). The only exceptions were: i) Maxim® and triclopyr which showed sporadically, a few brown-red necrotic lesions, when used at 1 mM of active principle. The severity of these lesions was ranked not higher than 1 (out of 7) and was always subtracted from the rating of disks compound-treated and inoculated with Psa. ii) Leaf curling sometimes appeared in IAA and NAA-treated disks. Phytotoxicity-related necrosis was also not associated with the use of Silwet L-77 and the dissolvent agents, namely DMSO, aceton and NaOH, at the concentration used.
The compounds produced three patterns of necrotic reactions compared with NT-Psa controls: i) substantial equivalence or an (slight) increase/decrease in the necrosis index which was not statistically significant (this indicates a lack of a differential effect, i.e. a Neutral Effect compared with NT-Psa, NE); ii) a statistically significant decrease in the necrosis index derived from a delayed and less intense symptom expression (Decrease and Delay of symptom Expression, DDE); iii) a statistically significant increase in the necrosis index derived from an earlier and more intense symptom expression (Increase and Anticipation of symptom Expression, IAE). Hereafter we use NE, DDE and IAE to refer to these responses.
Fosetyl-aluminum, BABA and BIT produced a DDE (Fig. 4). Testing multiple concentrations of Fosetyl-aluminum and BABA clearly revealed a dose-dependent response namely a progressive decrease in the necrosis index associated with an increase in concentration (Figs. 4 and 5). These effects were also confirmed on Hayward (Online Resource 5).
Statistically significant decrease in the necrosis index of leaf disks (cv. Belen) treated with Fosetyl-aluminum, BABA (DL-β-Amino-n-butyric acid) and BIT (saccharin) in independent experiments and subsequently inoculated with Pseudomonas syringae pv. actinidiae (Psa). The comparison is with NT-Psa, i.e. the control non-treated and inoculated with Psa. Necrosis indexes marked by different letters are significantly different from each other (p < 0.05). The numbers indicate the independent statistical analyses conducted separately on data from two temporally-distinct recordings: the first in grey, the second black. The vertical bars indicate the standard error of the means
MeJA and Maxim® and its active principle, triclopyr, conferred the IAE response (Fig. 6, Online Resource 6). Maxim® and triclopyr were tested with multiple concentrations: 0.01 mM and 0.03 mM (label recommendations) and 0.2 mM and 1 mM, which were chosen to simulate in vitro an illicit overdose of phytoregulator application in the plantations. A dose-dependent response was evident with a progressive increase in IAE associated with the illicit concentrations.
Statistically significant increase in the necrosis index of leaf disks (cv. Belen) treated with MeJA (Methyl jasmonate), Maxim® and its active principle triclopyr in independent experiments and subsequently inoculated with Pseudomonas syringae pv. actinidiae (Psa). The comparison is with NT-Psa, i.e. the control non-treated and inoculated with Psa. Necrosis indexes marked by different letters are significantly different from each other (p < 0.05). The numbers indicate the independent statistical analyses conducted separately on data from two temporally-distinct recordings: the first in grey, the second black. The vertical bars indicate the standard error of the means
NE was recorded from all the other tested compounds. Online Resource 7 contains the graphs of the necrosis index of IAA, NAA, ACC, SA, GA, PBZ and turmeric extract (Curcuma longa). For other NE-conferring compounds, the necrosis index was repeatedly higher – BTH and kinetin – or lower – isotianil – than the NT-Psa, though not significantly (Online Resource 8). BTH was tested using a concentration series including 0.475 mM (= 20 g/hl of the commercial product, which was recommended in the how-to-use label for use on kiwifruit). The mean value of the necrosis index increased in a dose-dependent manner and was always higher than that of NT-Psa.
Bacterial dynamic growth evaluation by real-time PCR in Fosetyl-aluminum-treated leaf disks
Real-Time PCR analysis showed that the amount of Psa DNA (measured as NoPQ, Normalized relative Pathogen Quantity) was higher in NT-Psa than in Fosetyl-aluminum-Psa disks for each time-point examined, with differences progressively increasing over time. These differences were statistically significant for time-points 72 and 144 h (first experiment) and time-point 144 h (second experiment) (Fig. 7). This analysis also shows a progressive increase in NoPQ in NT-Psa disks over time, supported by statistical significance (time-points 72 and 144 h vs all the others in the first experiment and time-point 144 h vs all the others in the second experiment), while no increases were recorded in the Fosetyl-aluminum-Psa disks (Fig. 7). All differences were detected with 0.0067 ≥ p ≥ 0.00014.
Pseudomonas syringae pv. actinidiae (Psa) growth dynamics monitored by Real-Time PCR in Fosetyl-aluminum and water-treated leaf disks, at different time-points (TP, hours). Each point of the curves represents the Normalized relative Pathogen Quantity (NoPQ) at a certain TP. The vertical bars indicate the standard error of the means. Significant comparisons in the I experiment were: TP-72 Fosetyl-aluminum vs TP-72 Water, p = 0.00014, and TP-144 Fosetyl-aluminum vs TP-144 Water, p = 0.00014; in the II experiment: TP-144 Fosetyl- aluminum vs TP-144 Water, p = 0.00014
Greenhouse plant assay with Fosetyl-aluminum and BTH
Testing the phytotoxicity of the compounds showed that plant viability was not at risk. Plants treated with Fosetyl-aluminum did not differ from untreated plants. Plants treated with BTH showed some foliar chlorosis starting from 10 days after the first treatment. The oldest leaves became cardboard-like. In addition, after 1 month there was some reduction in growth compared with water and Fosetyl-aluminum-treated plants.
We performed three inoculation trials, trial A (bacterial suspension sprayed on the leaves) and trials B1 and B2 (bacterial suspension syringe-injected in the stem). With regard to the inoculated plants in trial A, soon after winter dormancy, i.e. after 10 months from the inoculation, nine out of 12 plants in the untreated group and all the BTH-treated plants had lost their viability and failed to re-sprout. On the other hand, three plants in the group treated with Fosetyl-aluminum were dead. At the end of the observation period, June 2017 (third year) none of the untreated inoculated plants survived, whereas seven plants treated with Fosetyl-aluminum still survived and in normal vegetative condition (Fig. 8; Table 3).
In vivo trials conducted in the greenhouse (a) and open field (b, c) to confirm the effects of Fosetyl-aluminum and BTH on the infection of Pseudomonas syringae pv. actinidiae (Psa) (a inoculation; b, c natural infection). For the greenhouse trials, we report the disease severity index for each experimental group (McKinney 1923) (untreated control in grey, Fosetyl-aluminum in azure, BTH in green). For the field trials, we report the disease incidence meant as percentage (expressed as frequency) of symptomatic leaves on the whole canopy (b) and the average number of active cankers per tree (c). Concentrations of Fosetyl-aluminum expressed in Kg/hl and in mM (b and c) refer to the commercial product and the active principle respectively. The vertical bars indicate standard error of the means. Differences were significant with p < 0.05
In trials B1 and B2, after inoculation, small bark cankers appeared on the stem of all the experimental groups inoculated with Psa, near the infiltration points and then later in more distant points. After winter dormancy (i.e. 9 months post inoculation) and until the end of the trials, the three inoculated experimental groups were affected differently by sprout stunting, microphilia, and death of the epigeal part or of the whole plant. Plants treated with BTH were the most severely affected and all or nearly all had lost their viability at the time of final recording (Fig. 8; Table 3). On the other hand, in plants treated with Fosetyl-aluminum no deaths occurred and only two plants from trial B2 lost the viability of the epigeal part, but in any case produced normal-looking suckers 2 weeks later (Online resource 9). Disease severity in the untreated plants was intermediate between the BTH and the Fosetyl-aluminum experimental groups.
In the survivors, Psa was detected by Real-Time PCR and remained undetected by isolation on nutritive medium. All non-inoculated controls kept viability.
Fosetyl-aluminum-based field trials
At start of the trial, Psa was detected in the orchards by both isolation and Real-Time PCR in trees outside of the 30-tree experimental plots. At this point, no evident symptoms had appeared on the plants of the plots under observation. Throughout the trial, Psa was always detected concomitantly with symptom appearance, leaf spotting and canker on stem and twigs. In field 1, we recorded both leaf spotting and active cankers on stem and twigs. In field 2, only cankers were recorded, in fact the trees had large canopies with dense foliage which coalesced thus preventing leaf evaluation and attribution to each tree. Three months after starting the trial, leaf spotting incidence in field 1 was significantly lower in the groups treated with Fosetyl-aluminum than in the control. During the observation period, one tree in the control group showed abundant red exudates and sunken bark and lost its viability in July. Similarly, in both orchards the average number of cankers per tree was significantly lower in the groups treated with Fosetyl-aluminum than in the control. Differences were significant below the 0.01 level. No significant differences were detected in the comparison between the two groups treated with different doses of Fosetyl-aluminum. Figure 8 shows the results of the last recordings (8 July) (the intermediate was similar, data not shown). The weather conditions during the field trial are given in Online Resource 10.
Discussion
Our investigation into the direct inhibitive effects of the compounds on Psa in in vitro assays gave different results depending on the compound examined. The inhibition patterns helped us in assessing whether any DDE response obtained with leaf disk assay might be due also to a direct inhibitive effect. Although the plant system (the disks or the whole plant) or the medium may possibly be able to tune the inhibitive effect, a match, albeit not perfect, between the action of the compound on the bacterium in a medium and in the plant tissue is generally well accepted in the literature. In fact, in relation to Psa and similarly to what we performed, Scortichini (2014) associated the bactericidal action of chitosan on Psa, assessed in vitro, with a reduction in disease symptoms in kiwifruit treated with chitosan.
The leaf disk assay, developed in this work, was time-, space- and labour-saving, highly repeatable and robust as it was free from any unwanted effect such as a precocious senescence of the leaf tissue or phytotoxicity.
We were developing our leaf disk assay at the same time as another research group was developing its own leaf disk assay comparing the virulence of Psa strains by inoculating leaf disks and whole plants (Prencipe et al. 2018). Their leaf disk assay was highly reliable as the results fitted those of in vivo tests with a high correlation coefficient. Similarly, in our study we confirmed the reliability of our leaf disk assay in representing the plant response relating to Fosetyl-aluminum and BTH, two compounds with opposing actions. Interestingly, a correlation between in vitro and in vivo assays also emerged for MeJA and SA, which conferred an IAE and NE response in our leaf disk assay, respectively, in line with in planta results of Cellini et al. (2014) on a yellow fleshed cultivar. Nevertheless, the rationale of our leaf disk assay is to select compounds conferring the DDE and the IAE response on disk, which is the first step of a procedure aimed at launching specific confirmatory trials in planta. Regarding the compound screening activity, all data here obtained reasonably suggest that at least some of these increased/decreased the necrosis index by stimulating a response by the host. In addition, some hormones/compounds that are well known for their involvement in resistance signalling, revealed a direct and inhibitive effect on bacterial in vitro growth. For example, SA strongly decreased bacterial growth in the range 1–8 mM, with a high inhibition, 87.6%, already at a relatively low concentration, 2 mM (Fig. 1, Online Resource 3). These results suggest that the effect of resistance inducers on the resistance response cannot be correctly evaluated without assessing their direct effect on microorganism growth and viability.
Cellini et al. (2014) reported a decrease in disease severity caused by Psa on A. chinensis var. deliciosa seedlings after spray treatment with SA at 2.5 mM. Based on our results this concentration exerts a strong inhibitive effect on in vitro growth of Psa (Fig. 1), thus making it challenging to differentiate in planta the direct antibacterial effect from a genuine induction of resistance.
We found that BABA conferred a DDE response in the leaf disks. BABA is a hormone-like non-proteinogenic amino acid (Baccelli and Mauch-Mani 2017, Balmer et al. 2019) and can prime the plant for a faster and stronger activation of stress-specific defence mechanisms upon exposure to stress. In our study any direct inhibitive effect of BABA on Psa was ruled out in vitro. Subject to the specifications made above on the possible tuning capacity of the medium or the plant tissue, we suggest that the DDE response in leaf disks was probably due to resistance induction. We believe that this highlights the importance to start in vivo trials.
Phosphites, PHI (H2PO3−, HPO32−), are water-mixed phosphorous acid (H3PO3) neutralized with different cations. Fosetyl-aluminum is a phosphite species neutralized with aluminum ions thus forming aluminum tris (O-ethyl phosphonate) (McDonald et al. 2001). PHI species are phloem and xylem mobile and are especially known for their use in plant protection against oomycete, fungi and bacteria (Gómez-Merino and Trejo-Téllez 2015; Achary et al. 2017). PHI exert a dose-dependent dual mode of action: disease resistance induction and direct inhibition of the pathogen. As a resistance inducer, PHI is able to activate defence mechanisms in the pre-challenge phase and/or to prime defence for an augmented response after pathogen attack (Eshragi et al. 2011; Massoud et al. 2012; Dalio et al. 2014). In this work, we showed that Fosetyl-aluminum has a dose-dependent toxic/biocidal effect on Psa in vitro (Fig. 1). Our study highlighted that the antibacterial efficacy derived from both the acidifying capacity of the compound and additional characteristic(s) inherent in the structure of the molecule. This confirms that PHIs have different direct modes of action and thus multiple target sites within the microbial cell (Achary et al. 2017).
In the leaf disk assay, similarly to the trend observed in in vitro bacterial growth, we recorded a dose-dependent reduction in necrosis by Fosetyl-aluminum, in line with what reported above for PHI. This was also linked to a strong reduction in bacterial colonization, as verified by Real-Time PCR. Not only leaf disk assays, but also trials with potted plants and in the open field, clearly showed that Fosetyl-aluminum can significantly control bacterial canker.
The fact that Psa was detected in surviving treated plants by Real-Time PCR, but not by isolation, could be due to the efficacy of Fosetyl-aluminum in restricting bacterial colonization in plants under a threshold detectable by isolation, which is a less sensitive method compared with Real-Time PCR. It seems likely that Psa viability is decreased in planta by Fosetyl-aluminum, as in in vitro conditions (this work). Alternatively, there may have been an induction of a viable-but-not-culturable (VNBC) state for Psa as a consequence of a stress response, even though to the best of our knowledge this has never been reported for Psa.
In experiments conducted under controlled conditions, Monchiero et al. (2015) reported that Fosetyl-aluminum provided some protection in kiwifruit against Psa. They used the compound at a much lower concentration than we did, i.e. 0.16 g/l of the active principle, corresponding to 0.36 mM. In our study, this concentration had almost no toxic effect on Psa (Fig. 1), thus we speculate that they could have obtained primarily a specific effect of induction of resistance. Given that both modes of action of PHI are dose-dependent, an increase in the dose likely also increases the protection efficacy.
Whilst our results suggest that Fosetyl-aluminum acts in Actinidia by exerting a direct inhibitive/biocidal effect on Psa, this does not necessarily mean it is the only mode of action. Interestingly, priming and defence activation induced by PHI varied depending on the pathosystem, thus involving different hormonal signalling pathways and defence responses, but also complex hormonal signatures (SA, JA, ET) (Eshragi et al. 2011; Massoud et al. 2012; Dalio et al. 2014; Burra et al. 2014).
JA is a lipid-derived hormone involved in plant development and in mediating plant response to biotic and abiotic stress (Wasternack and Strnad 2016). In our study, the effects of MeJA – an increase in susceptibility to Psa in the leaf disks, in line with in planta results by Cellini et al. (2014) – were probably mediated by the plant response as the in vitro growth of the bacterium was not affected by this compound. This indicates that this hormone is likely a susceptibility inducer that disrupts the existing hormonal homeostasis in the basal resistance to Psa, which is inefficient in itself to counteract the bacterium, thus increasing susceptibility even further.
The growing awareness that the hormone IAA plays a pivotal role in a trade-off between growth and defence and significantly increase susceptibility to (emi-)biotrophs (Robert-Seilaniantz et al. 2011; Kazan and Lyons 2014; Ludwig-Muller 2015; Naseem et al. 2015), prompted us to test the effect of this hormone in the leaf disk assay. It is well known that pathogens, including P. syringae, manipulate auxin metabolism in order to activate it, thus promoting susceptibility (Cui et al. 2013, Kunkel and Harper 2018). It is also worth mentioning that Flores et al. (2018) demonstrated that Chilean Psa-3 strains all produce auxin at different levels and hypothesized that they may play a role in disease development.
In this work, we tested the effect of IAA and additional synthetic auxins, NAA and triclopyr (a chlorinated auxin) and its commercial formulate, Maxim®, which is widely used in kiwi orchards to increase fruit size. While treatments with a relatively high concentration of IAA and NAA – 1 mM – did not influence the outcome of the assay, Maxim® and triclopyr increased the necrosis index significantly at a five times lower concentration, 0.2 mM. This concentration is higher than recommended on the product label, thus simulating an illicit overdose to increase crop yield. The difference between triclopyr and the other auxins suggests that it has a much higher biological activity.
Similarly to our results, exogenous applications of natural and synthetic auxins have been shown to enhance disease susceptibility of Arabidopsis thaliana to P. syringae pv. tomato (Pst) DC 3000 (Navarro et al. 2006; Chen et al. 2007; Gonzáles-Lamothe et al. 2012).
These results deserve further investigation in order to clear if a fraudulent enhancement of IAA-like phytoregulator application in the orchards might significantly impair basal resistance of kiwi, thus adding to previous resistance-breaking events due both to the low genetic variability of the host and to pathogen evolution (McCann et al. 2017).
We tested the effects of SA, its main analogue BTH, and other synthetic molecules mimicking a subset of known SA functions – PBZ, BIT and isotianil (Faize and Faize 2018).
SA is a major stress hormone with key roles in different layers of the plant immune response, i.e. Pattern Triggered Immunity (PTI), Effector Triggered Immunity (ETI), and Systemic Acquired Resistance (SAR) (Zhang and Li 2019). BTH is the most studied resistance inducer mimicking SA. It soon replaced SA due to its adverse effects on plants, although its own action was still affected by phytotoxicity (Faize and Faize 2018).
Results reported by Cellini et al. (2014), Michelotti et al. (2018) and De Jong et al. (2019) on kiwifruit-Psa suggest that some resistance responses are elicited by BTH. In fact, following BTH treatment and Psa inoculation, they all reported a decrease in leaf spotting and in the multiplication rate of bacterial cells, and the activation of signalling components and defence responses traceable to the SA pathway. Nevertheless, no final outcome – resistance/survival vs susceptibility/death – was investigated.
Our results on salicylates seem to contradict data from Cellini et al. (2014), Michelotti et al. (2018) and De Jong et al. (2019), as we identified a NE response in the leaf disk assay, except for BIT which caused a DDE response. Specifically, BTH caused a dose-dependent increase in the necrosis index – though not statistically supported – that however was fully confirmed by the hypersusceptible reactions obtained in BTH-treated potted plants. It is worth noting that the results by these authors are not comparable with ours. Cellini et al. (2014) monitored symptom development over 21 days in artificially-infected seedlings of yellow and green-fleshed Actinidia varieties – age and dimension were not reported. Thus, the final outcome of the inoculation in terms of symptom development and death pattern, in a time frame longer than 21 days, was not reported. In addition, in this time frame, they found a clear decrease in bacterial canker symptoms by BTH in plants of A. chinensis var. deliciosa, while only milder effects were obtained on A. chinensis var. chinensis. Moreover, SA-treated A. chinensis var. chinensis did not respond with a decrease in disease severity but instead had a neutral effect (Cellini et al. 2014), which is in line with our results on leaf disk assay, although at a much lower dose.
Marcelletti et al. (2011) and McCann et al. (2013) provided evidence of variety-specific differences in the growth dynamics of different Psa biovars in green and yellow-fleshed kiwi trees. They reported an enhanced growth and spread of the pandemic lineage Psa-3 on A. chinensis var. chinensis (cv. Hort16A) relative to its performance on A. chinensis var. deliciosa (cv. Hayward). On the other hand, Psa-2 performed better on cv. Hayward than on cv. Hort16A. This is clearly suggestive of the specific adaptation of Psa-3 to yellow-fleshed kiwifruit.
Similarly, to Cellini et al. (2014), De Jong et al. (2019) recorded a decrease in leaf spotting in BTH-treated A. chinensis var. deliciosa plantlets by monitoring leaf symptoms in a time window not larger than 19/28 days. Interestingly, endophytic Psa populations in BTH-treated plants were not significantly lower than in the untreated control until 96 h after inoculation (De Jong et al. 2019).
Michelotti et al. (2018) monitored Psa multiplication in in vitro plantlets of A. chinensis var. chinensis in the first 48 h of infection, showing a strong reduction in the bacterium titer in BTH-treated plantlets compared with water-treated plantlets. However, the final outcome of the inoculation in terms of symptom development and death pattern was not reported.
Both Michelotti et al. (2018) and Cellini et al. (2014) used BTH at 1.7 mM, which is a 3.6 fold higher concentration than the maximum concentration admitted for field application, 20 g/hl Bion® i.e. 0.475 mM BTH, which we used in our experiments.
Unlike the papers cited above, we recorded disease symptoms of the inoculated plants over a long period, until the complete death of the most susceptible experimental plots. Thus, we considered survival/death, rather than leaf spotting, as the phenotypic marker for resistance evaluation. Although our approach is time consuming, it is necessary when the resistance level of any host plant to any lethal disease has to be evaluated. Based on this type of evaluation, all three of our experiments on potted plants confirmed that BTH treatment promoted disease development and mortality compared with untreated control inoculated with Psa.
Several hypotheses can suggest why BTH increased susceptibility in long-term experiments, which involve its phytotoxicity and/or hijacking strategies which P. syringae pathovars are able to enact i.e. by taking advantage of the host response to enhance their virulence (Gonzalez-Lamothe et al. 2012).
It could be questioned why phytotoxicity affected BTH-treated plants whereas it was not in evidence in BTH-treated leaf disks. This was due, in all probability, to the short exposure time of leaf disks compared with potted plants, which presumably absorbed the compound effectively with their root system.
Conclusions
The overall results of our study clearly suggest that triclopyr, MeJA and BTH induce susceptibility to Psa in the leaf disk assay, which was also confirmed in greenhouse trials for BTH.
Importantly our study also highlighted that some compounds are useful – Fosetyl-aluminum – or potentially useful – BABA and BIT – for bacterial canker control. Based on literature data and our own experimental data, the potential of these compounds to induce resistance against Psa should be investigated further. In fact, exploiting host basal resistance with resistance inducers, in addition to their direct inhibitive effects, is a sustainable approach for reducing the impact on the environment of other dangerous products.
References
Achary, V. M. M., Ram, B., Manna, M., Datta, D., Bhatt, A., Reddy, M. K., & Agrawal, P. K. (2017). Phosphite: A novel P fertilizer for weed management and pathogen control. Plant Biotechnology Journal, 15, 493–1508. https://doi.org/10.1111/pbi.12803.
Antoniacci, L., Bugiani, R., Rossi, R., Cavazza, F., Franceschelli, F., & Scannavini, M. (2014). Impiego di prodotti di sintesi e naturali nella difesa dal cancro batterico del kiwi (Pseudomonas syringae pv. actinidiae). Atti Giornate Fitopatologiche, 2, 173–180.
Baccelli, I., & Mauch-Mani, B. (2017). When the story proceeds backward: The discovery of endogenous β-aminobutyric acid as the missing link for a potential new plant hormone. Communucative & Integrative Biology, 10(2). https://doi.org/10.1080/19420889.2017.1290019.
Balestra, G. M., Mazzaglia, A., Spinelli, R., Graziani, S., Quattrucci, A., & Rossetti, A. (2008). Cancro batterico su Actinidia chinensis. L’Informatore Agrario, 38, 75–76.
Balmer, A., Glauser, G., Mauch-Mani, B., & Baccelli, I. (2019). Accumulation patterns of endogenous β-aminobutyric acid during plant development and defence in Arabidopsis thaliana. Plant Biology, 21(2), 318–325. https://doi.org/10.1111/plb.12940.
Burra, D. D., Berkowitz, O., Hedley, P. E., Morris, J., Resjo, S., Levander, F., Liljeroth, E., Andreasson, E., & Alexandersson, E. (2014). Phosphite-induced changes of the transcriptome and secretome in Solanum tuberosum leading to resistance against Phytophthora infestans. BMC Plant Biology, 14, 254. https://doi.org/10.1186/s12870-014-0254-y.
Butler, M. I., Stockwell, P. A., Black, M. A., Day, R. C., Lamont, I. L., & Poulter, R. T. M. (2013). Pseudomonas syringae pv. actinidiae from recent outbreaks of kiwifruit bacterial canker belong to different clones that originated in China. PLoS ONE, 8(2), e57464. https://doi.org/10.1371/journal.pone.0057464.
Cameron, A., & Sarojini, V. (2014). Pseudomonas syringae pv. actinidiae: Chemical control, resistance mechanisms and possible alternatives. Plant Pathology, 63, 1–11. https://doi.org/10.1111/ppa.12066.
Cellini, A., Fiorentini, L., Buriani, G., Yu, J., Donati, I., Cornish, D. A., Novak, B., Costa, G., Vanneste, J. L., & Spinelli, F. (2014). Elicitors of the salicylic acid pathway reduce incidence of bacterial canker of kiwifruit caused by Pseudomonas syringae pv. actinidae. Annals of Applied Biology, 165, 441–453. https://doi.org/10.1111/aab.12150.
Chapman, J. R., Taylor, R. K., Weir, B. S., Romberg, M. K., Vanneste, J. L., Luck, J., & Alexander, B. J. R. (2012). Phylogenetic relationships among global populations of Pseudomonas syringae pv. actinidiae. Phytopathology, 102, 1034–1044. https://doi.org/10.1094/PHYTO-03-12-0064-R.
Chen, Z., Agnew, J. L., Cohen, J. D., He, P., Shan, L., Sheen, J., & Kunkel, B. N. (2007). Pseudomonas syringae type III effector AvrRpt2 alters Arabidopsis thaliana auxin physiology. Proceedings of the National Academy of Sciences, 104, 20131–20136. https://doi.org/10.1073/pnas.0704901104.
Colombi, E., Straub, C., Künzel, S., Templeton, M. D., McCann, H. C., & Rainey, P. B. (2017). Evolution of copper resistance in the kiwifruit pathogen Pseudomonas syringae pv. actinidiae through acquisition of integrative conjugative elements and plasmids. Environmental Microbiology, 19(2), 819–832. https://doi.org/10.1111/1462-2920.13662.
Corsi, B., Linthorst, J. M. H., Forni, C., & Riccioni, L. (2017). Enhancement of PR1 and PR5 gene expressions by chitosan treatment in kiwifruit plants inoculated with Pseudomonas syringae pv. actinidiae. European Journal of Plant Pathology, 148, 163–179. https://doi.org/10.1007/s10658-016-1080-x.
Cui, F., Wu, S., Sun, W., Coaker, G., Kunkel, B., He, P., & Shan, L. (2013). The Pseudomonas syringae type III effector AvrRpt2 promotes pathogen virulence via stimulating arabidopsis auxin/indole acetic acid protein turnover. Plant Physiology, 162, 1018–1029. https://doi.org/10.1104/pp.113.219659.
Dalio, R. J. D., Fleischmann, F., Humez, M., & Osswald, W. (2014). Phosphite protects Fagus sylvatica seedlings towards Phytophthora plurivora via local toxicity, priming and facilitation of pathogen recognition. PLoS One, 9(1), e87860. https://doi.org/10.1371/journal.pone.0087860.
De Jong, H., Reglinski, T., Elmer, P. A. G., Wurms, K., Joel, L., Vanneste, J. L., Guo, L. F., & Alavi, M. (2019). Integrated use of Aureobasidium pullulans strain CG163 and Acibenzolar-S-methyl for management of bacterial canker in kiwifruit. Plants, 8(8), 287. https://doi.org/10.3390/plants8080287.
Eshragi, L., Anderson, J., Aryamanesh, N., Shearer, B., Mc Comb, J., Hardy, G. E. S. J., & O'Brien, P. A. (2011). Phosphite primed defence responses and enhanced expression of defence genes in Arabidopsis thaliana infected with Phytophthora cinnamomi. Plant Pathology, 60, 1086–1095. https://doi.org/10.1111/j.1365-3059.2011.02471.x.
Faize, L., & Faize, M. (2018). Functional analogues of salicylic acid and their use in crop protection. Agronomy, 8, 5. https://doi.org/10.3390/agronomy8010005.
Ferrante, P., & Scortichini, M. (2009). Identification of Pseudomonas syringae pv. actinidiae as causal agent of bacterial canker of yellow kiwifruit (Actinidia chinensis Planchon) in Central Italy. Journal of Phytopathology, 157, 768–770. https://doi.org/10.1111/j.1439-0434.2009.01550.x.
Ferrante, P., & Scortichini, M. (2010). Molecular and phenotypic features of Pseudomonas syringae pv. actinidiae isolated during recent epidemics of bacterial canker on yellow kiwifruit (Actinidia chinensis) in Central Italy. Plant Pathology, 59(5), 954–962. https://doi.org/10.1111/j.1365-3059.2010.02304.x.
Ferrante, P., & Scortichini, M. (2015). Redefining the global populations of Pseudomonas syringae pv. actinidiae based on pathogenic, molecular and phenotypic characteristics. Plant Pathology, 64, 51–62. https://doi.org/10.1111/ppa.12236.
Flores, O., Prince, C., Nuñez, M., Vallejos, A., Mardones, C., Yañez, C., Besoain, X., & Bastías, R. (2018). Genetic and phenotypic characterization of indole-producing isolates of Pseudomonas syringae pv. actinidiae obtained from chilean kiwifruit orchards. Frontiers in Microbiology, 9, 1907. https://doi.org/10.3389/fmicb.2018.01907.
Fratarcangeli, L., Rossetti, A., Mazzaglia, A., & Balestra, G. M. (2010). Il ruolo del rame nella lotta al cancro batterico del kiwi. L'Informatore Agrario, 8, 52–56.
Fujikawa, T., & Sawada, H. (2016). Genome analysis of the kiwifruit canker pathogen Pseudomonas syringae pv. actinidiae biovar 5. Scientific Reports, 6, 21399. https://doi.org/10.1038/srep21399.
Fujikawa, T., & Sawada, H. (2019). Genome analysis of Pseudomonas syringae pv. actinidiae biovar 6, which produces the phytotoxins, phaseolotoxin and coronatine. Scientific Reports, 9, 3836. https://doi.org/10.1038/s41598-019-40754-9.
Gallelli, A., L’Aurora, A., & Loreti, S. (2011). Gene sequence analysis for the molecular detection of Pseudomonas syringae pv. actinidiae: Developing diagnostic protocols. Journal of Plant Pathology, 93(2), 425–435. https://doi.org/10.4454/jpp.v93i2.1198.
Gallelli, A., Talocci, S., Pilotti, M., & Loreti, S. (2014). Real-time and qualitative PCR for detecting Pseudomonas syringae pv. actinidiae isolates causing recent outbreaks of kiwifruit bacterial canker. Plant Pathology, 63(2), 264–276. https://doi.org/10.1111/ppa.12082.
Gómez-Merino, F. C., & Trejo-Téllez, L. I. (2015). Biostimulant activity of phosphite in horticulture. Scientia Horticulturae, 196, 82–90. https://doi.org/10.1016/j.scienta.2015.09.035.
Gonzalez-Lamothe, R., El Oirdi, M., Normand Brisson, N., & Kamal, B. K. (2012). The conjugated auxin indole-3-acetic acid–aspartic acid promotes plant disease development. The Plant Cell, 24, 762–777. https://doi.org/10.1105/tpc.111.095190.
Han, H. S., Nam, H. Y., Koh, Y. J., Hur, J. S., & Jung, J. S. (2003). Molecular bases of high-level streptomycin resistance in Pseudomonas marginalis and Pseudomonas syringae pv. actinidiae. The Journal of Microbiology, 41(1), 16–21.
Kazan, K., & Lyons, R. (2014). Intervention of Phytohormone pathways by pathogen effectors. The Plant Cell, 26(6), 2285–2309. https://doi.org/10.1105/tpc.114.125419.
Koh, Y. J., Cha, J. B., Chung, J. H., & Lee, H. D. (1994). Outbreak and spread of bacterial canker in kiwifruit. Korean Journal of Plant Pathology, 10, 68–72.
Kunkel, B. N., & Harper, C. P. (2018). The roles of auxin during interactions between bacterial plant pathogens and their hosts. Journal of Experimental Botany, 69(2), 245–254. https://doi.org/10.1093/jxb/erx447.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods, 25(4), 402–408. https://doi.org/10.1006/meth.2001.1262.
Ludwig-Müller, J. (2015). Bacteria and fungi controlling plant growth by manipulating auxin: Balance between development and defense. Journal of Plant Physiology, 172, 4–12. https://doi.org/10.1016/j.jplph.2014.01.002.
Marcelletti, S., Ferrante, P., Petriccione, M., Firrao, G., & Scortichini, M. (2011). Pseudomonas syringae pv. actinidiae draft genomes comparison reveal strain-specific features involved in adaptation and virulence to Actinidia species. PLoS ONE, 6(11), e27297. https://doi.org/10.1371/journal.pone.0027297.
Massoud, K., Barchietto, T., Le Rudulier, T., Pallandre, L., Didierlaurent, L., Garmier, M., Ambard-Bretteville, F., Seng, J. M., & Saindrenan, P. (2012). Dissecting phosphite-induced priming in arabidopsis infected with Hyaloperonospora arabidopsidis. Plant Physiology, 159, 286–298. https://doi.org/10.1104/pp.112.194647.
Mazzaglia, A., Studholme, D. J., Taratufolo, M. C., Cai, R., Almeida, N. F., Goodman, T., Guttman, D. S., Vinatzer, B. A., & Balestra, G. M. (2012). Pseudomonas syringae pv. actinidiae (PSA) isolates from recent bacterial canker of kiwifruit outbreaks belong to the same genetic lineage. PLoS ONE, 7(5), e36518. https://doi.org/10.1371/journal.pone.0036518.
McCann, H. C., Rikkerink, E. H. A., Bertels, F., Fiers, M., Lu, A., Rees-George, J., Andersen, M. T., Gleave, A. P., Haubold, B., Wohlers, M. W., Guttman, D. S., Wang, P. W., Straub, C., Vanneste, J., Rainey, P. B., & Templeton, M. D. (2013). Genomic analysis of the kiwifruit pathogen Pseudomonas syringae pv actinidiae provides insight into the origins of an emergent plant disease. PLoS Pathogens, 9(7), e1003503. https://doi.org/10.1371/journal.ppat.1003503.
McCann, H. C., Li, L., Liu, Y., Li, D., Pan, H., Zhong, C., Rikkerink, E. H. A., Templeton, M. D., Straub, C., Colombi, E., Rainey, P. B., & Huang, H. (2017). Origin and evolution of the kiwifruit canker pandemic. Genome Biology and Evolution, 9(4), 932–944. https://doi.org/10.1093/gbe/evx055.
McDonald, A. E., Grant, B. R., & Plaxton, W. C. (2001). Phosphite (phosphorous acid): Its relevance in the environment and agriculture and influence on plant phosphate starvation response. Journal of Plant Nutrition, 24(10), 1505–1519. https://doi.org/10.1081/PLN-100106017.
McKinney, H. H. (1923). Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. Journal of Agricultural Research, 26, 195–225.
Michelotti, V., Lamontanara, A., Buriani, G., Orrù, L., Cellini, A., Donati, I., Vanneste, J. L., Cattivelli, L., Tacconi, G., & Spinelli, F. (2018). Comparative transcriptome analysis of the interaction between Actinidia chinensis var. chinensis and Pseudomonas syringae pv. actinidiae in absence and presence of acibenzolar-S-methyl. BMC Genomics, 19(1), 585. https://doi.org/10.1186/s12864-018-4967-4.
Monchiero, M., Gullino, M. L., Pugliese, M., Spadaro, D., & Garibaldi, A. (2015). Efficacy of different chemical and biological products in the control of Pseudomonas syringae pv. actinidiae on kiwifruit. Australasian Plant Pathology, 44, 13–23. https://doi.org/10.1007/s13313-014-0328-1.
Navarro, L., Dunoyer, P., Jay, F., Arnold, B., Dharmasiri, N., Estelle, M., Voinnet, O., & Jones, J. D. G. (2006). A plant miRNA contributes to antibacterial resistance by repressing Auxin signaling. Science, 312(5772), 436–439. https://doi.org/10.1126/science.1126088.
Naseem, M., Srivastava, M., Tehseen, M., & Ahmed, N. (2015). Auxin crosstalk to plant immune networks: A plant-pathogen interaction perspective. Current Protein and Peptide Science, 16(5), 389–394. https://doi.org/10.2174/1389203716666150330124911.
OEPP/EPPO. (2014). PM 7/120 (1) Pseudomonas syringae pv. actinidiae. OEPP/EPPO Bulletin, 44(3), 360–375. https://doi.org/10.1111/epp.12171.
Petriccione, M., Mastrobuoni, F., Zampella, L., & Scortichini, M. (2015). Reference gene selection for normalization of RT-qPCR gene expression data from Actinidia deliciosa leaves infected with Pseudomonas syringae pv. actinidiae. Scientific Reports, 5, 16961. https://doi.org/10.1038/srep16961.
Pizzinat, A., Giordani, L., Asteggiano, L., Nari, L., Giraudo, M., Pavarino, A., Bevilacqua, A., Spinelli, F., Morone, C., & Vittone, G. (2014). Contenimento della batteriosi dell’actinidia in Piemonte. Atti Giornate Fitopatologiche, 2, 163–172.
Prencipe, S., Gullino, M. L., & Spadaro, D. (2018). Pseudomonas syringae pv. actinidiae isolated from Actinidia chinensis Var. deliciosa in northern Italy: Genetic diversity and virulence. European Journal of Plant Pathology, 150, 191–204. https://doi.org/10.1007/s10658-017-1267-9.
Pucci, N., Orzali, L., Modesti, V., Lumia, V., Brunetti, A., Pilotti, M., & Loreti, S. (2018). Essential oils with inhibitory capacities on Pseudomonas syringae pv. actinidiae, the causal agent of kiwifruit bacterial canker. Asian Journal of Plant Pathology, 12(1), 16–26. https://doi.org/10.3923/ajppaj.2018.16.26.
Quattrucci, A., Renzi, M., Rossetti, A., Ricci, L., Taratufolo, C., Mazzaglia, A., & Balestra, G. M. (2010). Cancro batterico del kiwi verde: nuove strategie di controllo. L'Informatore Agrario, 16, 53–58.
Robert-Seilaniantz, A., Grant, M., & Jones, J. D. G. (2011). Hormone crosstalk in plant disease and defense: More than just JASMONATE-SALICYLATE antagonism. Annual Review of Phytopathology, 49, 317–343. https://doi.org/10.1146/annurev-phyto-073009-114447.
Scortichini, M. (1994). Occurrence of Pseudomonas syringae pv. actinidiae in Italy. Plant Pathology, 43, 1035–1038. https://doi.org/10.1111/j.1365-3059.1994.tb01654.x.
Scortichini, M., Marcelletti, S., Ferrante, P., Petriccione, M., & Firrao, G. (2012). Pseudomonas syringae pv. actinidiae: A reemerging, multi-faceted, pandemic pathogen. Molecular Plant Pathology, 13(7), 631–640. https://doi.org/10.1111/j.1364-3703.2012.00788.x.
Scortichini, M. (2014). Field efficacy of chitosan to control Pseudomonas syringae pv. actinidiae, the causal agent of kiwifruit bacterial canker. European Journal of Plant Pathology, 140, 887–892. https://doi.org/10.1007/s10658-014-0515-5.
Simonetti, G., Pucci, N., Brasili, E., Valletta, A., Sammarco, I., Carnevale, E., Pasqua, G., & Loreti, S. (2019). In vitro antimicrobial activity of plant extracts against Pseudomonas syringae pv. actinidiae causal agent of bacterial canker in kiwifruit. Plant Biosystems, 154(1), 100–106. https://doi.org/10.1080/11263504.2019.1699194.
Takikawa, Y., Serizawa, S., Ichikawa, T., Tsuyumu, S., & Goto, M. (1989). Pseudomonas syringae pv. actinidiae pv. Nov.: The causal bacterium of canker of kiwifruit in Japan. Japan Journal of Phytopathology, 55(4), 437–444. https://doi.org/10.3186/jjphytopath.55.437.
Tosi, L., Tacconi, G., Spinelli, F., Posenato, G., Bertaiola, F., & Giacopini, A. (2014). Efficacia di alcuni formulati nei confronti del cancro batterico dell’actinidia causato da Pseudomonas syringae pv. actinidiae. Atti Giornate Fitopatologiche, 2, 157–162.
Valente, M., Ortugno, C., Tosi, L., Scannavini, M., Pelliconi, F., Fagioli, L., Scortichini, M., Vittone, G., Fiorillo, E., Pradolesi, G., & Donati, G. (2014). Bion®50WG (acibenzolar-S-methyl), induttore delle autodifese della pianta: efficacia nella prevenzione di Pseudomonas syringae pv. actinidiae su Actinidia. Atti Giornate Fitopatologiche, 2, 147–156.
Vanneste, J. L. (2017). The scientific, economic, and social impacts of the New Zealand outbreak of bacterial canker of kiwifruit (Pseudomonas syringae pv. actinidiae). Annual Review of Phytopathology, 55, 377–399. https://doi.org/10.1146/annurev-phyto-080516-035530.
Vavala, E., Passariello, C., Pepi, F., Colone, M., Garzoli, S., Ragno, R., Pirolli, A., Stringaro, A., & Angiolella, L. (2016). Antibacterial activity of essential oils mixture against PSA. Natural Product Research, 30, 412–418. https://doi.org/10.1080/14786419.2015.1022543.
Wasternack, C., & Miroslav Strnad, M. (2016). Jasmonate signaling in plant stress responses and development – Active and inactive compounds. New Biotechnology, 33(5), 604–613. https://doi.org/10.1016/j.nbt.2015.11.001.
Zhang, Y., & Li, X. (2019). Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Current Opinion in Plant Biology, 50, 29–36. https://doi.org/10.1016/j.pbi.2019.02.004.
Acknowledgements
This research was supported by: i) a Mipaaf (the Italian Ministry of Agricultural and Forestry Policies) project, “INTERACT/ARDICA, Interventi di coordinamento ed implementazione alle azioni di ricerca, lotta e difesa al cancro batterico dell’Actinidia”; ii) a Latium Region PSR (Programma di Sviluppo Rurale) project, “PRO.ACTI.BA.RE, Protezione dell’actinidia dal cancro batterico (Pseudomonas syringae pv. actinidiae) mediante l’uso di induttori di resistenza”.
Availability of data and material (data transparency)
Raw data can be viewed upon request.
Code availability (software application or custom code)
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
The authors have no conflict of interest or competing interests to declare.
Ethics approval
All principles of ethical and professional conduct have been followed during this research and the drafting of this manuscript.
Consent to participate
All the authors gave consent to participate in the authorship.
Consent for publication
All the authors agreed with the content of the article and gave explicit consent to submit and publish the article.
Electronic supplementary material
Online Resource 1
Details on the preparation of compounds to be tested for the anti-bacterial activity and for their effect on the infection of Pseudomonas syringae pv. actinidiae (Psa) in the leaf disk assays (PDF 110 kb)
Online Resource 2
Symptom scoring for the inference of the disease severity index of the greenhouse trials (McKinney 1923) and data on the outcome of the trials. A. Classes of symptoms observed on potted plants treated with Fosetyl-aluminum, BTH and water and inoculated with Pseudomonas syringae pv. actinidiae (Psa), and the attributed scores. The score derived from the combination of different classes was obtained from the sum of the values of each class. Class combinations including F are valued as F. B. Score assigned to each plant within each treatment and trial (A, B1 and B2) based on symptom class ranking. (PDF 68 kb)
Online Resource 3
Optical density (OD) of Pseudomonas syringae pv. actinidiae (Psa) after growing in NSB supplemented with compounds/molecules, which were also used in the leaf disk assay. All measurements were made with a spectrophotometer at 660 nm after 20–22 h incubation, when non- compound-supplemented Psa cultures (called Control 1, C1) reached approximately the value of 1.0. A: OD measurements for each compound/molecule at different concentration. The comparison is with C1. Control 2 (C2) is the absorbance of non-inoculated NSB. Different letters indicate significant differences between groups (p < 0.01). The vertical bars indicate standard deviations of the means. Asterisks indicate the bactericidal concentrations. B: compounds/molecules and concentrations that caused a growth reduction ≥40% compared with C1. Differences are expressed as growth percentage compared with C1 (= 100%) and as percentage growth reduction (PDF 161 kb)
Online Resource 4
Example of non-inoculated leaf disks used as the control in order to assess any wound, time and compound-related senescence/phytotoxicity. The figure includes the non-treated leaf disks (NT) and leaf disks treated twice with BTH, following the protocol assay. Ten leaf disks were treated with BTH suspensions at different concentrations, and the experiment was repeated three times. We show close-ups of five disks for each concentration. The image was taken 20 days after BTH treatments. Belen was used as the source of the leaf disks (PDF 268 kb)
Online Resource 5
Confirmation of the outcome of the leaf disk assay on Actinidia chinensis var. deliciosa cv. Hayward: significant decrease in the necrosis index of leaf disks treated with Fosetyl-aluminum and BABA and subsequently inoculated with Pseudomonas syringae pv. actinidiae (Psa). The comparison is with the control non-treated and inoculated with Psa (NT-Psa). Necrosis indexes marked by different letters are significantly different from each other [p < 0.01 (Fosetyl-aluminum), p < 0.05 (BABA)]. The numbers indicate the independent statistical analyses conducted separately on data from two temporally-distinct recordings: the first in grey, the second black. The vertical bars indicate the standard error of the means (PDF 34 kb)
Online Resource 6
The IAE response (Increase and Anticipation of symptom Expression) obtained by treating the leaf disks with triclopyr and MeJA at various concentrations and then inoculating with Pseudomonas syringae pv. actinidiae (Psa). NT-Psa = non-treated and inoculated with Psa; NT = non-treated and non-inoculated (PDF 318 kb)
Online Resource 7
Examples of compounds/molecules whose treatment on leaf disks (cv. Belen) did not influence the outcome of Pseudomonas syringae pv. actinidiae (Psa) inoculation in terms of the necrosis index. The comparison is with the control non-treated and inoculated with Psa (NT-Psa). Necrosis index values marked by different letters are significantly different from each other (p < 0.05). The numbers indicate the independent statistical analyses conducted separately on data from two temporally-distinct recordings: the first in grey, the second black. The vertical bars indicate the standard error of the means (PDF 37.4 kb)
Online Resource 8
Compounds/molecules whose treatment on leaf disk (cv. Belen) did not statistically differ from the control non-treated and inoculated with Pseudomonas syringae pv. actinidiae (Psa) (NT-Psa) in terms of necrosis index, similarly to those depicted in Online resource 7. Unlike the effects reported in Online resource 7, the mean values of the necrosis index in this figure were repeatedly and evidently lower or higher than the NT-Psa in all the experiments. This prompts a hypothesis on a fine tuning by these compounds in the direction of resistance/susceptibility induction. Necrosis indexes marked by the different letters are significantly different from each other (p < 0.05). The numbers indicate the independent statistical analyses conducted separately on data from two temporally-distinct recordings: the first in grey, the second black. The vertical bars indicate the standard error of the means. Concentrations of BTH expressed in mM and g/l refer to the active principle and the commercial product (Bion) respectively (PDF 34.3 kb)
Online Resource 9
Healthy-looking Fosetyl-aluminum-treated/Psa-inoculated plants (a) compared with BTH-treated/Psa-inoculated plants (b), which were dead or severely stunted (B2 trial, during spring/summer 2016) (PDF 175 kb)
Online Resource 10
Rainfall (gray line) and average (green), maximum (red) and minimum (azure) temperature during the field trial. The trial was conducted from 9 April to 8 July (PDF 91 kb)
Rights and permissions
About this article
Cite this article
Brunetti, A., Pucci, N., Modesti, V. et al. In vitro and in planta screening of compounds for the control of Pseudomonas syringae pv. actinidiae in Actinidia chinensis var. chinensis. Eur J Plant Pathol 158, 829–848 (2020). https://doi.org/10.1007/s10658-020-02119-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-02119-1