Abstract
Proline/hydroxyproline-rich glycoprotein (P/HRGP) level in pearl millet genotypes resistant to downy mildew increase after inoculation with the oomycete pathogen Sclerospora graminicola. Using purified P/HRGPs from pearl millet cell walls, polyclonal antibodies (Pab-P/HRGP) were raised in rabbit. Based on this antiserum, an enzyme immunoassay was developed that displays a linearity detection range from 0.01 to 10 μg P/HRGP. Western blot analysis, confirming the induction of three marker P/HRGPs in the infected resistant genotype, and immunocytochemical studies on P/HRGP localization either in epidermal peelings or in suspension-cultured cells demonstrated the specificity of the antiserum. Besides its characterization, Pab-P/HRGP was employed to screen various genotypes of pearl millet for fast, sensitive and specific detection of induced P/HRGPs upon infections. The results presented are discussed with presumed importance to downy mildew disease and the use of this new antiserum in pearl millet screening for disease resistance.
Similar content being viewed by others
Introduction
Plant cell walls are dynamic structures that change with physiological fluctuations as well as upon external stimuli from the environment. The most abundant structural proteins in plant cell walls are hydroxyproline-rich glycoproteins (HRGPs). They are induced in disease-resistant responses, specifically in incompatible plant–pathogen interactions (Davis et al. 1997). There is evidence that HRGPs act as impenetrable physical barriers for pathogen ingress. Functionally they contribute to the mechanical properties of the cell wall by creating a network formed by glycoprotein elements and in addition they contribute to plant defence, by strengthening the wall upon pathogen attack or mechanical wounding. They may immobilize the pathogens by ionically binding to their negatively charged surfaces because of their positive charge (Leach et al. 1982; Mazau et al. 1987; Cassab and Varner 1988; Sommer-Knudsen et al. 1998).
HRGPs in dicot species have been more widely studied than in monocots. Probably the best studied HRGP from monocotyledonous plants is the one from maize, which has been characterized at protein, cDNA and genomic levels (Garcia Muniz et al. 1998). Other examples where these cell wall components have been studied are HRGPs in wheat after Fusarium culmorum infection (Kang and Buchenauer 2003) and HRGPs in pearl millet (Pennisetum glaucum) after Sclerospora graminicola infection (Shailasree et al. 2004; Deepak et al. 2007a, b).
Earlier studies from this laboratory have purified and characterized the HRGPs involved in pearl millet defence against downy mildew disease caused by S. graminicola. The isolated protein had a pI of 9.8 and exhibited molecular masses of 27, 17, and 14 kDa on SDS-PAGE (Deepak et al. 2007a). The amino acid composition analysis revealed high amounts of proline, hydroxyproline and serine. Cross-reactivity with the monoclonal antibody MAC 265, a rat monoclonal antibody raised against a 95-kDa glycoprotein involved in the pea–Rhizobium symbiosis (Vandenbosch et al. 1989), and the presence of a signature amino acid sequence, PVYK, strongly suggested the purified glycoprotein to be a member of P/HRGPs class (Deepak et al. 2007a).
The use of defined antibodies has been described as one of the most direct approaches available to gain an understanding of bio-molecular mechanisms including those involved in plant–pathogen interactions and can be used as fast, sensitive, and specific tool to detect infected plants and resistance (Smallhood et al. 1995; Willets et al. 2000; Kang and Buchenauer 2003). The aim of the present investigation was to generate a polyclonal antiserum directed against P/HRGPs which accumulate in pearl millet seedlings during the resistance reaction against downy mildew. This antiserum was utilised in various immunological techniques such as ELISA, western blotting, and immunocytochemical studies to detect induced HRGPs in pearl millet challenged with the downy mildew pathogen.
Materials and methods
Plant material
Pearl millet (P. glaucum) cultivars showing various degrees of downy mildew disease incidence (DMDI) upon inoculation with the pathogen S. graminicola under field conditions were used in the study. The cultivars used were IP18292, IP18293, IP18296, IP18297 [(highly resistant (HR) with <5% DMDI)]; UCC17, 841B [(resistant (R) with >5–<10% DMDI)]; 5141B, UGC23 [(susceptible (S) with 11–25% DMDI)] and 23B, HB3, 7042S (highly susceptible (HS) with >25% DMDI) and were obtained from the ‘All India Coordinated Pearl Millet Improvement Project’ (AICPMIP), Jodhpur, Rajasthan, India and the International Crop Research Institute in Semi Arid Tropics (ICRISAT), Patencheru, India.
Pathogen and inoculum preparation
The phytopathogenic oomycete S. graminicola, isolated from pearl millet cv. 7042S and maintained on the same cultivar under greenhouse conditions was used for all inoculation experiments. Leaves of pearl millet showing profuse sporulation of S. graminicola on the abaxial side were collected in the evening from plants maintained under greenhouse conditions (25–30°C, >95% RH). Collected leaves were thoroughly washed under running tap water to remove the previous crop of sporangia. The leaves were then blotted dry, cut into small pieces, and kept in a moist chamber for sporulation. The next morning the fresh crop of sporangia was harvested into sterile distilled water. For use as inoculum, the zoospore concentration was adjusted to 4 × 104 ml−1 with a haemocytometer.
Inoculation and sample collection
Seeds were germinated on discs of moist blotter paper in Petri dishes at 25 ± 2°C for 2 days. A zoospore suspension of 4 × 104 ml−1 was prepared and used to root-dip inoculate 2 day-old seedlings (Safeeulla 1976). The inoculated seedlings were harvested at 8 h post-inoculation (h.p.i) and stored at −20°C for subsequent analysis.
Antigen preparation
Isolation of cell wall proteins P/HRGP
Cell wall proteins were extracted from coleoptiles of pearl millet seedlings as reported by Leach et al. (1982). All procedures were carried out at 4°C. Coleoptiles from the seedlings were homogenized in 0.5 M potassium phosphate buffer, pH 7.0. They were repeatedly washed with the same buffer followed by distilled water and by centrifuging at 10,000×g for 10 min at 4°C. The resulting pellet was suspended in three volumes of ethanol: 1.25 N HCl (1:1). After 2 days at 4°C, cellular debris was removed by centrifugation. Protein was precipitated by adding 3 vol of cold acetone and incubated at 4°C overnight. The precipitated protein was centrifuged at 10,000×g for 15 min. Acetone was decanted and the pellet was air-dried. The acetone precipitate containing the total cell wall proteins was dissolved in 0.05 M sodium acetate buffer (pH 3.5) and used for all future experiments.
Purification of P/HRGPs
P/HRGPs from the total cell wall proteins were purified by Sephadex G-200 column chromatography and reverse-phase HPLC (Deepak et al. 2007a). The purified protein was analyzed by native acidic and SDS PAGE and was directly used as antigen for immunization of rabbits for production of polyclonal antiserum against P/HRGPs.
Antiserum production
Antiserum was raised in New Zealand white rabbit following the standard immunization protocol (Harlow and Lane 1988). For immunization, the pure protein in phosphate buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Phosphate Buffer, pH 7.2) mixed with Freund’s incomplete adjuvant (1:1) was injected at multiple sites through intramuscular routes. Three such injections were given at an intermittent interval of one week by increasing the concentration of protein (100–150 μg protein); 500 μg of protein was used in total. A booster dose was given at the fourth week and the rabbit was test bled after 4 days. The rabbit was further boosted twice up to 6 weeks in a similar way. The blood was collected 2 days after the last injection and was allowed to clot at room temperature for 30 min followed by 2–3 h at 4°C. The antiserum separated from blood serum was centrifuged at 200×g for 15 min and stored at −20°C.
Enzyme-linked immunosorbent assay (ELISA)
Determination of antibody titer of the Polyclonal antiserum- P/HRGP (Pab-P/HRGP)
Direct ELISA was used to detect the titer of the antiserum and to test the specificity for HRGP of pearl millet. ELISA was carried out in commercially available 96-well microtiter plates (Nunc, Denmark). Antigen (10 μg) was loaded to each well of the ELISA plate and the volume was made up to 100 μl/well with antigen buffer (sodium acetate buffer, pH 3.6, 10 mM). Plates were incubated overnight at RT. They were washed with 200 μl/well ELISA wash buffer (PBS containing 0.5% Tween-20). To reduce non-specific binding, the wells were blocked with 200 μl of ELISA blocking buffer (PBS containing 5% skimmed milk powder) for 1 h at 37°C. After washing, the wells were loaded with 100 μl of polyclonal antiserum at various dilutions ranging from 1:100 to 1:20,000 in ELISA dilution buffer (PBS containing 0.1% BSA). The plate was incubated for 1 h at 37°C. After washing, the second antibody goat anti-rabbit IgG conjugated with horseradish peroxidase (Bangalore Genei Pvt. Ltd., Bangalore, India) was added at 1:20,000 dilution in ELISA dilution buffer and incubated for 1 h at 37°C. The conjugated enzyme was detected by addition of the substrate o-phenylenediamine at 0.04% (100 μl/well) in PBS containing 0.02% H2O2. The reaction was incubated at RT for 10 min and stopped by adding 10 μl of 1 M H2SO4. The developed colour was read at 490 nm using a microtiter plate reader (Spectramax 340PC 384, Molecular Devices Corporation, Sunnyvale, CA, USA). The ELISA reactivity was examined and the maximum dilution showing maximum reactivity was considered as the antibody titer.
Evaluation of ELISA sensitivity for pure and crude antigen for Pab-P/HRGP
Sensitivity of the raised polyclonal antibody to both pure and crude antigen was assessed using ELISA assays. The ELISA test protocol followed was similar to that described earlier (Clark and Adams 1977). Different protein concentration of 0.01, 0.1, 0.5, 1, 2, 4, 6, 8, and 10 μg of both pure P/HRGPs and the total cell wall proteins were used for the analysis. To investigate the specificity of Pab-P/HRGP, various proteins (10 μg of myosin, β-galactosidase, phosphorylase-b, BSA, ovalbumin, carbonic anhydrase) were used instead of P/HRGPs and their cross-reactivity was determined.
Western blot analysis
About 40 μg of protein were subjected to 12% SDS PAGE. The separated proteins were blotted onto polyvinylidene difluoride (PVDF) membrane following Winston et al. (1987) using Multiphor II (Pharmacia, Freiburg, Germany) electrophoretic transfer apparatus according to the manufacturer’s protocol. The blots were blocked in 2% fat-free milk in Tris-buffered saline (TBS: 10 mM Tris HCl, pH 8.0 containing 150 mM NaCl). They were then probed with Pab-P/HRGP (1:5,000). Subsequently, the blots were incubated with the second antibody, anti-rabbit IgG horseradish peroxidase-conjugate, at 1:10,000 dilution for 1 h at RT. After washing twice with TBST (TBS plus 0.5% Tween-20) for 5 min each, the blots were stained for peroxidase activity with 1.33 mM 3,3′-diaminobenzedine (DAB) and 10 mM hydrogen peroxide.
Immunocytochemical localization
Localization of P/HRGPs in epidermal peelings
For immunocytochemical investigations, a protocol described by Thordal-Christensen et al. (1997) and modified by Mellersh et al. (2002) was used. Epidermal peelings from the coleoptile region of the two day-old pearl millet cvs 7042S and IP18296 at 8 h after inoculation with S. graminicola were used. Epidermal peelings from coleoptiles of seedlings treated with distilled water served as the uninoculated control. Peelings were fixed and decolorized in boiling 95% ethanol and submerged in 1% SDS for 3 days at 80°C to remove soluble proteins. Blocking was carried out by incubation of the epidermal peelings with 1% BSA in TBS for 30 min followed by three washings with TBS. The peelings were incubated for 2 h at 37°C with primary antibodies diluted in TBS buffer (1:5,000). After washing three times with TBS, they were incubated with the second antibody, goat anti-rabbit IgG horseradish peroxidase-conjugate (1:10,000 dilution), for 1 h at 37°C. After washing, the epidermal peelings were stained for peroxidase activity with 2.7 mM DAB and 10 mM hydrogen peroxide. Specificity of labelling was assessed by a control assay, which involved incubation of the epidermal peelings with secondary antibody alone and omitting the primary antibody step or by using the preimmunoserum. Areas of cell wall with protein interaction were visualized under the microscope (Wild Leitz, Germany) at different magnifications of 500- and 1,250-fold.
Localization in suspension cell culture
The pearl millet cell culture was raised from susceptible (7042S) and resistant (IP18296) cultivars by following the method of Vasil and Vasil (1981). The well-established suspension cells were regularly sub-cultured onto fresh medium at 1:5 dilution rates at 10-day intervals and after 10 sub-cultures the cells were used for the study. Cell culture (108 cells ml−1) at the mid-point of log phase of growth (16 day old) was used for the experiment. The culture was filtered through Mira cloth, which retains intact cells and inoculated with zoospores (4 × 104 spores ml−1) of S. graminicola. They were harvested 8 h.p.i. by filtering through Mira cloth and intact cells were used for the study. The cells were washed thoroughly in distilled water. Immediately, cells were incubated for 2 h at 37°C with Pab-P/HRGP diluted 1:5,000 in TBS buffer. After washing three times with TBS, they were incubated with the second antibody for 1 h at 37°C. The suspension cells were stained for peroxidase activity with 2.7 mM DAB and 10 mM hydrogen peroxide. Suspension cells treated with distilled water served as the uninoculated control.
Determination of P/HRGP in resistant and susceptible pearl millet genotypes by ELISA
Two day-old seedlings of highly resistant (IP18292, IP18293, IP18296, IP18297), resistant (UCC17, 841B), highly susceptible (23B, HB3, 7042S) and susceptible (5141B, UGC23) pearl millet genotypes were used for the study. Seedlings of all genotypes were inoculated with S. graminicola zoospores. Seedlings treated with distilled water served as the control. They were harvested at 8 h.p.i. Total cell wall proteins were extracted following the procedure described by Leach et al. (1982) and Shailasree et al. (2004). About 10 μg of total cell wall proteins were used for ELISA analysis with Pab-P/HRGP antibodies at 1:5,000 dilutions.
Results
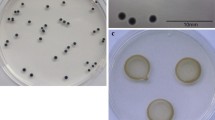
Polyclonal antiserum against P/HRGP was obtained after immunization of rabbits. Antibody sensitivity and the titer were determined using ELISA. A titer value of 1:5,000 was determined to provide sufficient reactivity with the antigen and hence was used for all future experiments. The Ouchterlony double diffusion with purified P/HRGP showed a single sharp precipitin band indicating the specificity of the antigen–antibody reaction while a smeared precipitin band was observed when the antibody reacted with the total cell wall proteins (Fig. 1). To determine the sensitivity of the antiserum, increasing amounts of purified P/HRGP and crude cell wall extracts were analyzed by ELISA (Fig. 2). The detection limit was determined to be 0.1 μg of P/HRGP. A fivefold higher sensitivity of purified over-crude P/HRGPs was observed. Up to the tested protein amount of 10 μg the response did not reach saturation (Fig. 2).
Ouchterlony double diffusion test to check the purity of the P/HRGPs with polyclonal antibodies (Pab-P/HRGP). Pab-P/HRGP (well 2) reactivity was analyzed with purified P/HRGP in well 1 and crude total cell wall extract P/HRGP in well 3. The white precipitin line formed by antigen–antibody reaction with purified P/HRGP and crude P/HRGP is indicated by an arrow
Evaluation of ELISA sensitivity for pure P/HRGP and crude antigen for Pab-P/HRGP. The ELISA reactivity of pure P/HRGP (filled circles) and crude extract (filled squares) of resistant pearl millet seedlings was carried out using primary antibody at a dilution of 1:5,000 and the ELISA plate was read at 490 nm using a microtiter plate reader
To investigate the specificity of the generated antiserum, several control experiments were performed: the preimmunoserum did not show cross-reactivity with HRGP; the presence of the horseradish peroxidase-conjugate linked secondary antibody was required to detect cross-reactive material. The anti-HRGP antiserum showed no cross-reaction with a number of unrelated proteins including myosin, β-galactosidase, phosphorylase-b, BSA, ovalbumin, carbonic anhydrase in both ELISA and western blot experiments (results not shown).
In contrast, Western blot analysis of the total cell wall proteins extracted from susceptible and resistant cultivars using Pab-P/HRGPs showed specific interactions (Fig. 3) with the P/HRGPs identified in previous studies (Shailasree et al. 2004; Deepak et al. 2007a). The major protein of 17 kDa was recognized in cell wall extracts of both inoculated and uninoculated samples of IP18296 and 7042S cultivars of pearl millet. The second HRGP of 14 kDa was detected in the resistant cultivar with or without inoculation, while in the susceptible cultivar it was present only upon inoculation. The third HRGP of 27 kDa was observed with low intensity in the inoculated and control samples of both 7042S and IP18296 (Fig. 3). To identify the epitope of P/HRGP against which the antiserum was directed, 100 μg of purified P/HRGP was deglycosylated using trifluromethane sulphonic acid for 3–4 h on ice as described by Edge et al. (1981). Western blot analysis of deglycosylated protein using Pab-P/HRGP gave similar pattern of three major proteins as with the native glycosylated protein, indicating that the protein moiety is important for antiserum recognition. Pab-P/HRGP was further used for immunolocalization studies of HRGPs in epidermal peelings of pearl millet cvs 7042S and IP18296 and also in cultured cells. The labelling was prominent in the pathogen penetration regions of cell walls of IP18296 seedlings inoculated with S. graminicola (Fig. 4a) but not when the preimmune serum was used instead of Pab-P/HRGP (Fig. 4c). Similar prominent reaction of the antibodies was not observed in the susceptible cv. 7042S upon inoculation with the pathogen (Fig. 4b). Furthermore, uninoculated samples of IP18296 and 7042S showed marginal labelling with Pab-P/HRGP antibody (results not shown). Incubation of the epidermal peelings of uninoculated and inoculated 7042S and IP18296 cultivars of pearl millet with the secondary antibody alone did not result in labelling of the cellular structures (results not shown).
Immunoblotting for the detection of the polypeptides 27, 17 and 14 kDa of P/HRGP. Two day-old resistant (IP18296) and susceptible (7042S) pearl millet seedlings were inoculated with a zoospore suspension of S. graminicola (4 × 104 zoospores ml−1). Seedlings harvested at 8 h.p.i. were used for the P/HRGP extraction. The primary antibody was used at a dilution of 1:5,000. Protein (40 μg each lane) was used for Western blot analysis. Lane 1 resistant inoculated; lane 2 resistant control; lane 3 susceptible inoculated; lane 4 susceptible control
Immunocytochemical localization of P/HRGPs in cell walls with Pab-P/HRGP antibody in the epidermal peelings from the coleoptile region and suspension cells of pearl millet upon inoculation with S. graminicola. Prominent labelling with Pab-P/HRGP antibody at the cell wall is seen in highly resistant IP18296 cultivar (a) and not in highly susceptible 7042S (b) upon inoculation with S. graminicola, and no labelling can be observed with preimmune serum in IP18296 (c). Bar = 100 μm. Prominent labelling is observed in cell walls of suspension culture cells of highly resistant cv. IP18296 upon inoculation with the pathogen compared to the mock control. A marginally higher labelling of cell walls in the susceptible HB3 culture cells was observed upon inoculation with the pathogen relative to its control. d Resistant inoculated; e resistant control; f susceptible inoculated; g susceptible control. Bar = 50 μm
Immunocytochemical localization of P/HRGP in suspension cells was also carried out. Interaction with Pab-P/HRGP in the apoplastidic region of cell with intense staining of DAB was visualized. The cell wall stained prominently in the IP18296-inoculated sample compared to its distilled water control (Fig. 4d, e). However, in a susceptible cultivar (HB3) cells no significant difference in staining was observed between S. graminicola inoculated and distilled water control samples (Fig. 4f, g).
To utilize the newly developed antiserum for detection of downy mildew resistance in infected plants, diagnostic P/HRGPs levels were evaluated in different genotypes of pearl millet with varying degrees of resistance to the pathogen. The reactivity of Pab-P/HRGP with the HRGPs in control and downy mildew-inoculated pearl millet seedlings was evaluated. Significantly higher amount of reactivity was recorded for highly resistant cultivars which further increased upon S. graminicola inoculation. The reactivity increased to 0.9 to approximately 0.99 in these cultivars upon inoculation with the downy mildew pathogen compared to 0.6 to 0.7 as observed in the control. In the resistant cultivar, the reactivity of the antibody followed a similar trend as observed in the highly resistant cultivar. However in susceptible and highly susceptible cultivars the reactivity of HRGPs with the antibody decreased. Furthermore there was no significant increase in the reactivity upon downy mildew infection (Fig. 5).
Validity of Pab-P/HRGP in detection of different susceptible and resistant pearl millet genotypes after inoculation. Two day-old seedlings of different pearl millet genotypes were inoculated with S. graminicola zoospores (4 × 104 zoospores ml−1). The cultivars used were IP19292, IP18293, IP18296, IP18297 (highly resistant, HR); UCC17, 841B (resistant, R); 23B, HB3, 7042S (highly susceptible, HS) and 5141B, UGC23 (susceptible, S). The seedlings were harvested 8 h.p.i. and used for extraction of P/HRGP; 10 μg of each sample was subjected to ELISA using Pab-P/HRGP at 1:5,000 dilutions. The reaction was read at 490 nm using a microtiter plate reader. Disease incidence was recorded in the field for the various genotypes listed. Data are means (±SE) of three independent experiments with readings determined in triplicate. Means designated with the same letter in the control and inoculated are not significantly different according to Tukeys HSD test at P < 0.05
Discussion
Recent studies indicated an important role for P/HRGPs in pearl millet defence against the oomycetous downy mildew pathogen S. graminicola (Shailasree et al. 2004; Deepak et al. 2007b). These P/HRGPs have been biochemically characterized (Deepak et al. 2007a). The present study was undertaken to generate defined P/HRGP antiserum as a tool to study and recognize reinforcement of the pearl millet cell wall to S. graminicola infection. Several different immunological assays were employed in the present study to characterize the reactivity of Pab-P/HRGP with induced P/HRGPs in pearl millet seedlings as a response to S. graminicola infection.
ELISA-based studies indicated that the new Pab-P/HRGP is a robust antiserum, which is able to specifically react with crude plant cell wall extracts. Moreover, the antiserum had a wide detection range in combination with specific interactions of P/HRGPs, mainly the dominating protein species of 14, 17, and 27 kDa. The reactivity of these three protein bands with the monoclonal antibody MAC 265 has previously been reported from this laboratory (Shailasree et al. 2004; Deepak et al. 2007a, b). These monoclonal antibodies were raised against a 95-kDa matrix glycoprotein from infection threads present in the pea–Rhizobium symbiosis (Vandenbosch et al. 1989). MAC 265 has been successfully used to identify HRGPs in legumes (Rathbun et al. 2002; Olsson et al. 2002). Pab-P/HRGP has revealed recognition properties for the whole glycoprotein and the protein moiety alone, which was similar to the MAC 265 monoclonal antibody recognition (Deepak et al. 2007a).
Cooper et al. (1987) mentioned the existence of HRGP in two forms, soluble and insoluble. The soluble form of HRGP has been referred to as extensin precursors. The soluble form is insolubilized in the cell wall during morphogenesis of plant and in defence. Immunocytochemical studies using Pab-P/HRGP antibodies revealed the presence of cross-linked HRGPs in the cell wall. Suspension-cultured pearl millet cells were also employed for immunocytochemical localization since they provide a good in vivo model for analysing the role of HRGPs in resistance. The higher staining intensity in inoculated compared to uninoculated cells provide evidence for accumulation and insolubilization of P/HRGP by cross-linking in cell walls during pathogen attack (Deepak et al. 2007b).
The molecular architecture of plant cell proteins and direct functional analyses of P/HRGP are not readily available and thus, defined antibodies are powerful tools to gain insight into the spatial and temporal accumulation of these HRGPs in defence. Previous research work on P/HRGPs suggested the involvement of HRGPs in the successful defence against the phytopathogenic oomycete S. graminicola (Shailasree et al. 2004; Deepak et al. 2007b). A correlation was observed between the amount of hydroxyproline induced in coleoptiles of different cultivars of pearl millet to S. graminicola infection and host resistance. Similar results were obtained in the present study using the P/HRGP antiserum. A correlation between ELISA results and the resistance of the different cultivars was observed (Fig. 5). The data obtained demonstrated that in pearl millet resistance against downy mildew disease, a high level of P/HRGP in the cell wall correlated with low susceptibility to the disease and vice versa. Moreover, the Pab-P/HRGP reacted differentially with the different genotypes of pearl millet varying in degree of resistance to S. graminicola infection. An increase in the Pab-P/HRGP reactivity was recorded with increase in downy mildew resistance in pearl millet genotypes. Hence, the intensity of antiserum reaction with antigen could be applied to test the existence of variations in pearl millet genotypes.
In summary, we raised, characterized, and applied a polyclonal anti P/HRGP antiserum. The data presented in this paper indicate its usefulness for an easy and rapid detection of P/HRGPs in pearl millet cultivars and to estimate their resistance against downy mildew disease.
References
Cassab, G. I., & Varner, J. E. (1988). Cell wall proteins. Annual Review Plant Physiology Plant Molecular Biology, 39, 321–353.
Clark, M. F., & Adams, A. N. (1977). Characteristics of the microplate method of enzyme linked immunosorbent assay for the detection of plant viruses. Journal of General Virology, 34, 475–483.
Cooper, J. B., Chen, J. A., Van Holst, G.- J., & Varner, J. E. (1987). Hydroxyproline-rich glycoproteins of plant cell walls. Trends in Biochemical Science, 12, 24–27.
Davis, H. A., Daniels, M. J., & Dow, J. W. (1997). Induction of extracellular matrix glycoproteins in Brassica petioles by wounding and in response to Xanthomonas campestris. Molecular Plant-Microbe Interaction, 10, 812–820.
Deepak, S., Shailasree, S., Sujeeth, N., Kini, K. R., Shetty, H. S., & Mithöfer, A. (2007a). Purification and characterization of proline/hydroxyproline-rich glycoprotein from pearl millet coleoptiles infected with downy mildew pathogen Sclerospora graminicola. Phytochemistry, 68, 298–305.
Deepak, S., Shailasree, S., Kini, K. R., Hause, B., Shetty, H. S., & Mithöfer, A. (2007b). Role of hydroxyproline rich glycoproteins in resistance of pearl millet against downy mildew pathogen Sclerospora graminicola. Planta, 226, 323–333.
Edge, A. S. B., Faltynek, C. R., Hof, L., Reichert, L. E., & Weber, P. (1981). Deglycosylation of glycoproteins by trifluoromethanesulphonic acid. Analytical Biochemistry, 118, 131–137.
Garcia Muniz, N., Martinez-Isquierdo, J. A. M., & Puigodomenech, P. (1998). Induction of mRNA accumulation corresponding to gene encoding a cell wall hydroxyproline rich glycoprotein by fungal elicitors. Plant Molecular Biology, 38, 623–632.
Harlow, E. D., Lane, D. (1988). In Antibodies, a laboratory manual. New York: Cold Spring Harbor Laboratory, 724 p. ISBN 087693142.
Kang, Z., & Buchenauer, H. (2003). Immunocytochemical localization of cell wall-bound thionins and hydroxyproline-rich glycoproteins in Fusarium culmorum-infected wheat spikes. Journal of Phytopathology, 151, 120–129.
Leach, J. E., Cantrell, M. A., & Sequeira, L. (1982). Hydroxyproline-rich bacterial agglutinin from potato. Plant Physiology, 70, 1353–1358.
Mazau, D., Rumeau, D., & Esquerre-Tugaye, M. T. (1987). Molecular approaches to understanding cell surface interactions between plants and fungal pathogens. Plant Physiological Biochemistry, 25, 337–43.
Mellersh, D. G., Foulds, I. V., Higgins, V. J., & Heath, M. C. (2002). H2O2 plays different roles in determining penetration failure in three diverse plant–fungal interactions. Plant Journal, 29, 257–268.
Olsson, P. A., Kjellbom, P., & Rosendahl, L. (2002). Rhizobium colonization induced changes in membrane-bound and soluble hydroxyproline-rich glycoprotein composition in pea. Physiologia Plantarum, 114, 652–660.
Rathbun, E. A., Naldrett, M. J., & Brewin, N. J. (2002). Identification of a family of extensin-like glycoproteins in the lumen of Rhizobium-induced infection threads in pea root nodules. Molecular Plant-Microbe Interactions, 15, 350–359.
Safeeulla, K. M. (1976). Biology and control of the downy mildews of pearl millet, sorghum and finger millet p. 304. Mysore: Wesley Press.
Shailasree, S., Kini, K. R., Deepak, S., Kumudini, B. S., & Shetty, H. S. (2004). Accumulation of hydroxyproline-rich glycoproteins in pearl millet seedlings in response to Sclerospora graminicola infection. Plant Science, 167, 1227–1234.
Smallhood, M., Martin, H., & Knox, J. P. (1995). An epitope of rice threonine and HRGP is common to cell wall and hydrophobic plasma membrane glycoproteins. Planta, 196, 510–522.
Sommer-Knudsen, J., Bacic, A., & Clarke, A. E. (1998). Hydroxyproline-rich plant glycoproteins. Phytochemistry, 47, 483–497.
Thordal-Christensen, H., Zhang, Z., Wei, Y., & Collinge, D. B. (1997). Sub-cellular localization of H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant Journal, 11, 1187–1194.
Vandenbosch, K. A., Bradley, D. J., Knox, J. P., Perotto, S., Butcher, G. W., & Brewin, N. J. (1989). Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO Journal, 8, 335–341.
Vasil, V., & Vasil, I. K. (1981). Somatic embryogenesis and plant regeneration from suspension cultures of pearl millet (Pennesitum americanum). Annals of Botany, 47, 669–678.
Willets, W. G. T., King, C. G. S., McCarteny, L., Orfila, C., Marcus, S. E., & Knox, J. P. (2000). Making and using antibody probes to study plant cell walls. Plant Physiology and Biochemistry, 38, 27–36.
Winston, S., Fuller, S., & Huller, J. (1987). Western blotting. In E.M. Ausubel (Ed.)Current protocols in molecular biology (pp. 10.8.1–10.8.6). New York: Wiley.
Acknowledgements
The authors are grateful to the DANIDA-ENRECA for the facilities made available for the research programme. We also thank the Department of Science and Technology, Government of India and Indian Council of Agricultural Research, New Delhi and the Max-Planck-Society for financial support. DS would like to thank the German Academic Exchange Service (DAAD) for financial support to carry out research work at the Max-Planck-Institute for Chemical Ecology, Jena, Germany. SS acknowledges the financial support and the research grant received from the Department of Science and Technology, New Delhi, India under the SERC Fast Track Young Scientist Programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Deepak, S., Shailasree, S., Sujeeth, N. et al. Serodiagnosis of pearl millet resistance to downy mildew by quantitating cell wall P/HRGP using polyclonal antiserum Pab-P/HRGP. Eur J Plant Pathol 121, 77–85 (2008). https://doi.org/10.1007/s10658-007-9246-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-007-9246-1