Abstract
There is a male sex disadvantage in morbidity and mortality due to COVID-19. Proposed explanations to this disparity include gender-related health behaviors, differential distribution of comorbidities and biological sex differences. In this study, we investigated the association between sex and risk of severe COVID-19 while adjusting for comorbidities, socioeconomic factors, as well as unmeasured factors shared by cohabitants which are often left unadjusted. We conducted a total-population-based cohort study (n = 1,854,661) based on individual-level register data. Cox models was used to estimate the associations between sex and risk for severe COVID-19. We additionally used a within-household design and conditional Cox models aiming to account for unmeasured factors shared by cohabitants. A secondary aim was to compare the risk of COVID-19 related secondary outcomes between men and women hospitalized due to COVID-19 using logistic regression. Men were at higher risk for hospitalization (HR = 1.63;95%CI = 1.57–1.68), ICU admission (HR = 2.63;95%CI = 2.38–2.91) and death (HR = 1.81;95%CI = 1.68–1.95) due to COVID-19, based on fully adjusted models. However, the effect of sex varied significantly across age groups: Among people in their 50s, men had > four times higher risk of COVID-19 death. The within-household design did not provide any further explanation to the sex disparity. Among patients hospitalized due to COVID-19, men had an increased risk for viral pneumonia, acute respiratory distress syndrome, acute respiratory insufficiency, acute kidney injury, and sepsis which persisted in fully adjusted models. Recognition of the combined effect of sex and age on COVID-19 outcomes has implications for policy strategies to reduce the adverse effects of the disease.
Similar content being viewed by others
Introduction
Since the outbreak of Coronavirus disease 2019 (COVID-19) caused by the virus SARS-CoV-2 detected in November 2019 and declared a pandemic by the World Health Organization in March 2020, a growing body of evidence reveals male sex as an important risk factor for severe COVID-19 outcomes. Globally, men are overrepresented among hospitalizations, intensive care unit (ICU) admissions and deaths due to COVID-19, despite similar infection rates between sexes.[1,2,3,4] However, the underlying causes for the observed sex disparity in severe COVID-19 outcomes remains unknown.
Apart from old age and male sex, comorbidities, including widespread cardiovascular and endocrine diseases, are known risk factors for severe COVID-19.[5] As the prevalence of many of these diseases differ between sexes, underlying comorbidities and related life-style factors have been suggested as a plausible explanation for the excess risk among men. Gender-based differences in attitudes and behaviors, such as COVID-19 risk perception, healthcare seeking behavior, and compliance to behaviors to avoid infection and spread of the disease,[6, 7] have also been suggested to contribute to the sex disparity. There are moreover biological sex differences with impact on the immune system that may explain the male vulnerability to severe COVID-19.[8, 9] Women generally elicit stronger innate and adaptive immune responses towards antigens,[8, 10,11,12] resulting in differences in prevalence and outcomes between men and women for several infectious, inflammatory, and autoimmune diseases; Generally, men are more vulnerable to infectious diseases, whereas women are more likely to develop autoimmune diseases. Studies from previous coronavirus outbreaks revealed that men are at higher risk for Severe Acute Respiratory Syndrome (SARS)[13] and Middle East Respiratory Syndrome (MERS).[14] There is also evidence that women in general develop a more robust humoral and cell-mediated immune response and report more adverse effects towards vaccination than men.[15,16,17,18] Similarly, differences between sexes in measured immune responses among COVID-19 patients have also been identified. Male sex has been associated with higher levels of proinflammatory cytokines and chemokines such as IL-6, IL-8, IL-18 and CCL5, higher neutrophil-lymphocyte ratios, lower absolute lymphocyte counts and a more robust induction of non-classical monocytes, whereas female sex has been associated with a more robust T-cell activation.[9, 19, 20] Thus, differences in immune responses have been suggested to explain the excess risk of severe COVID-19 outcomes among men, although the cause and effect between immune response and disease severity is not fully clear.
Numerous studies have indicated that male sex is a significant risk factor for severe COVID-19. Many studies are based on large, aggregated data with limited ability to adjust for important confounders, whereas other studies are smaller, hospital-based, and often suffer from selection bias and limited generalizability. Therefore, the main aim of this large population-based cohort study was to examine the sex disparity in the risk of hospitalization, ICU admission and death due to COVID-19 by utilizing the ability to link several health and administrative registers containing individual-level data on COVID-19 outcomes, as well as important comorbidities and socioeconomic factors. To analyze the sex disparity in severe COVID-19 outcomes further, we additionally used a within-household design in a subpopulation of the cohort aiming to adjust for unobserved factors shared by individuals living in the same household. This includes housing-related living conditions such as many social, economic and lifestyle related factors which are often shared by cohabitants to a large extent but are often left unadjusted despite being a potential source of omitted variable bias. The within-household design also partly accounts for SARS-CoV-2 exposure since much of the virus transmission occurs within households. A secondary aim of this study was to compare the risk of severe secondary outcomes related to COVID-19 between men and women hospitalized due to COVID-19.
Methods
Study design
We conducted a population-based prospective cohort study to examine the association between sex and risk for severe outcomes in COVID-19 defined as hospitalization, ICU admission or death due to COVID-19. The eligible study population was defined as all individuals aged 18 and older who were living in the region of greater Stockholm, Sweden, on March 1, 2020 (n = 1,854,666). The region of greater Stockholm represents more than one fifth of the entire population in Sweden. Five individuals with missing information on date of death were excluded resulting in a final study population of 1,854,661 individuals.
Through the unique personal identity number assigned to all Swedish citizens and residents intending to stay at least one year in Sweden,[21] we were able to perform unambiguous linkage between several population-based health and administrative registers for each individual in the cohort. Each individual was followed from March 1, 2020, until date of outcome event (i.e., hospitalization, ICU admission, or death due to COVID-19), emigration from the region of greater Stockholm, date of death, or to the end of the study period, whichever occurred first. The end of study period was defined as May 16, 2021 for hospitalization and ICU admission due to COVID-19 and December 31, 2020 for death due to COVID-19.
We further analyzed a cohabitation cohort to compare the risk of severe COVID-19 outcomes between men and women living within the same household. This comparison controlled for unknown and unmeasured factors shared among individuals living in the same household that might confound the association between sex and risk of severe COVID-19. Individuals living together evidently share the same housing-related living conditions, as well as many other social, economic and lifestyle related factors to a large extent. Further, since much of the transmission of SARS-CoV-2 occur within households, individuals living together have a similar risk of SARS-CoV-2 exposure. To create our cohabiting cohort, we started with the 1,854,661 individuals included in the population-based cohort and proceeded to exclude 4,173 individuals (< 1%) with missing information on household-id, and 564,842 individuals (30%) who were not living together with another adult person of the opposite sex. Among the 1,285,646 individuals that remained, we only included the oldest opposite-sexed pair of individuals living within the same household, with a maximum age difference of 5 years. In this step, 526,714 individuals (28%) were excluded, which resulted in a cohabiting cohort consisting of 758,932 individuals.
Main outcomes
We considered three main COVID-19 related outcomes in this study: hospitalization, ICU admission and death due to COVID-19.
We derived information on hospitalizations due to COVID-19 from the Stockholm Regional Healthcare Data Warehouse (Vårdanalysdatabasen [VAL]) which provides information on all admissions to hospital as well as all healthcare visits in primary and secondary care (defined as specialist outpatient care).[22] Each record contains one primary diagnosis and up to nine additional diagnoses coded according to the International Classifications of Diseases (ICD), date of hospital admission and discharge or date of healthcare visit among others. We defined hospitalization due to COVID-19 as the first date of admission to hospital with COVID-19 (ICD-10 code U07.1 or U07.2) recorded as a primary diagnosis in the VAL-database.
Admissions to ICU were identified from the Swedish Intensive Care Registry (SIR), a national quality register for intensive care in Sweden including all ICUs in the region of greater Stockholm.[23, 24] We defined admission to ICU due to COVID-19 as a record in the SIR during a hospital episode with a COVID-19 diagnosis (ICD-10 code U07.1 or U07.2).
Data on deaths due to COVID-19 was retrieved from the cause of death register (CDR) held by the Swedish National Board of Health and Welfare. The CDR has nationwide coverage and contains information from death records including the underlying and contributory causes of death coded according to the ICD.[25] We defined death due to COVID-19 as a recorded diagnosis of COVID-19 (ICD-10 code U07.1 or U07.2) as the underlying or contributory cause of death in the CDR.
To assess the robustness of our results, we also used a more inclusive definition of COVID-19 related hospitalizations. This definition held that COVID-19 could be registered as either a primary or a secondary diagnosis. We also performed a similar robustness check regarding COVID-19 related deaths, using a more restrictive definition that held that COVID-19 had to be an underlying cause of death.
Independent variables
Several comorbid conditions or history of diseases are associated with an increased risk for severe COVID-19 outcomes. Thus, we attained information on disease history, including possible comorbidities, for all study participants by searching recorded diagnoses in the VAL-database for the following diseases with ICD codes within parentheses; essential hypertension (I10.9), ischemic heart diseases (I20-25), heart failure (I50), stroke (I60-63), chronic obstructive pulmonary disease (COPD) (J44), asthma (J45), type 2 diabetes (E11), obesity (E66), chronic kidney disease (N18), chronic liver disease (K70), and cancer (C00-C97). We described those having an aforementioned disease recorded as a primary or secondary diagnosis on at least one consultation in primary or secondary care or during hospitalization from five years prior study entry until end of study period as having a comorbidity. For cases identified with COVID-19, only diagnoses recorded before the first COVID-19 diagnosis were considered.
We derived data on educational attainment and disposable household income from the Longitudinal integrated database for health insurance and labor market studies (LISA) maintained by Statistics Sweden.[26] Highest educational attainment was categorized into four groups: primary schooling (9 years or fewer), secondary education (9–12 years), higher education (> 12 years), and missing. Non-missing disposable family income was grouped into tertiles, while a separate group was made for those with missing information. The first tertile contained the lowest third of the income distribution, and the third tertile contained the highest third of the income distribution.
Clinical outcomes of COVID-19
A second aim of this study was to compare outcomes in terms of diagnoses associated with severe illness due to COVID-19 between men and women hospitalized with COVID-19 as a main diagnosis. The following diagnoses were considered with ICD-10 codes within parentheses: viral pneumonia (J12.8-J12.9, J18.9), acute respiratory distress syndrome (ARDS) (J80.9), acute respiratory insufficiency (J96.0), acute kidney injury (N17), acute hepatic injury (K72.0, K72.9), acute cardiac injury (I21), sepsis (R65.1), septic shock (R57.2, R57.9), pulmonary embolism (I26), cerebral infarct (I63), embolism and thrombosis (I74, I80, I82). To be defined as a clinical outcome of COVID-19, the diagnosis had to be diagnosed during a hospital episode with COVID-19 as the main diagnosis. Cases with sequential admissions with a readmission within one day of the previous discharge were considered as one hospital episode to account for transfer of patients within and between hospitals.
Statistical analysis
We used Cox proportional hazard regression models to estimate hazard ratios (HRs) with 95% confidence internals (CIs) for the risk of severe outcomes in COVID-19 associated with sex, using time after the start of follow-up in days as the underlying time scale. We adjusted all models for age using restricted cubic splines with 5 knots and then sequentially added other potential confounders including comorbidities and socioeconomic factors. We also tested for interactions between age and sex on COVID-19 outcomes and estimated separate HRs for men compared to women for different age groups (< 40, 40–49, 50–59, 60–69, 70–79 and 80 + years). The proportional hazards assumption was assessed graphically using log–log plots and tested based on Schoenfeld residuals. To further examine whether the effect of sex varied during follow-up, we split the follow-up time between the first and second wave of the pandemic (end of August, 2020) and included an interaction term between sex and time-period in our models.
We repeated the main analyses in the cohabitation cohort, using a standard Cox proportional hazards model, with partially and fully adjusted models as described above. To account for unobserved characteristics shared by men and women living in the same household, we performed corresponding analyses using conditional Cox models, stratified by household id. This approach allows each pair of cohabitants to have an individual baseline hazard function, while at the same time allowing it to vary between pairs. An attenuation of an estimate suggests that household-related factors contribute to the association, while the persistence of an estimate suggests that the association between sex and risk for severe COVID-19 is independent from factors shared within households.
We used multivariate logistic regression to explore if outcomes, in terms of diagnoses associated with severe COVID-19, differed between men and women hospitalized due to COVID-19. All tests were 2-sided and p < 0.05 was considered statistically significant. All analyses were conducted using Stata Statistical Software: Release 14 (StataCorp LP, College Station, Texas).
Results
The study population consisted of 1,854,661 individuals, representing the whole adult population of Stockholm County, who were followed for hospitalizations and ICU admissions due to COVID-19 between March 1, 2020 and May 16, 2021 with an average follow-up time of 432 days. During 2,192,925 person-years under observation, 16,475 hospitalizations and 1,974 ICU admissions occurred. The study cohort was also followed for deaths due to COVID-19 between March 1, 2020 and December 31, 2021, with an average follow up time of 302 days amounting to a total of 1,531,833 person-years under observation. During this period 3,281 deaths due to COVID-19 occurred. Table 1 shows the distribution of population at risk, hospitalizations, ICU admissions and deaths due to COVID-19 for the variables used in our analyses. The incidence rate for hospitalization, ICU admission and death due to COVID-19 was higher in men, people with older age, comorbidities, low income, and low education level.
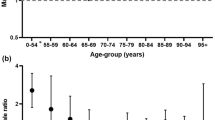
In Table 2, we present the results from multivariate Cox regressions of the risk for severe outcomes in COVID-19 among men compared to women. Male sex was associated with higher rates of hospitalization due to COVID-19, with HRs that remained stable around 1.6 in both partially and fully adjusted models (fully adjusted HR: 1.63; 95% CI: 1.57–1.68). However, there was a statistically significant (p < 0.05) interaction with age; men’s increased risk was most pronounced among middle aged people. Men aged 40–49 experienced a twice as large risk for hospitalization due to COVID-19 compared to women of the same age, while young adult men (18–39 years) only experienced 25% larger risk and men of old age (> 80 years) only experienced a 40% larger risk. The pattern was similar for ICU admissions due to COVID-19. Overall, men experienced approximately 2.6 times higher risk of ICU admission due to COVID-19, and the point estimate remained stable and statistically significant in all models (fully adjusted HR: 2.63; 95% CI: 2.38–2.91). There was again a statistically significant (p < 0.05) interaction with age. Young adult men (18–39 years) did not experience a statistically significant increased risk compared to women of the same age, whereas for all ages above 40, men experienced a three times larger risk for ICU admission. Finally, the pattern was also similar for death due to COVID-19. Overall, men experienced approximately 80% greater risk of death due to COVID-19 (fully adjusted HR: 1.81; 95% CI: 1.68–1.95). Again, this effect varied significantly (p < 0.05) across age groups with the most pronounced difference observed among men and women aged 50–59 years, where men experienced a ~ 4.6 times higher risk of death. The stark difference in risk of death then decreased with increasing age, settling at ~ 1.6 larger risk for men older than 80 years compared to women of the same age. We did not find any increased risk of death due to COVID-19 among men younger than 50 years of age, however, there were very few deaths in these age groups. Overall, adjusting for comorbidities and socioeconomic factors did not substantially change the point estimates for any of the outcomes; neither for the total cohort nor for any specific age group (Table 2).
Generally, we found no evidence that sex in models with hospitalizations and deaths related to covid-19 violated the proportional hazards assumption. However, in models with covid-19 related ICU admissions as the outcome, sex seemed to violate the proportional hazards assumption. This was addressed in sensitivity models including an interaction term between sex and time-period. Expectedly, there were no statistically significant interactions between sex and time-period (i.e., the first and second wave of the pandemic) for covid-19 related hospitalizations and deaths, and the HRs for sex were essentially the same during the two time-periods. For covid-19 related ICU admissions, the effect of male sex was slightly lower during the second wave (HR: 2.37; 95% CI: 2.08–2.71) compared to the first wave (HR: 2.98; 95% CI: 2.57–3.45). To assess the robustness of our results, we estimated all our models using a more inclusive definition of COVID-19 related hospitalizations. Reassuringly, our results using this definition of COVID-19 related hospitalization were similar with one exception; women at reproductive age (18–40 years) had an increased risk for hospitalization related to COVID-19 compared to men of the same age (S1 Table). However, this was solely explained by pregnant women receiving pregnancy and delivery-related health care as the main diagnosis and COVID-19 registered as a secondary diagnosis, which means that the difference in the results likely reflects intensive testing and reporting among this otherwise generally healthy female population.[27] We also performed a similar robustness check regarding COVID-19 related deaths, but with a more restrictive definition of deaths due to COVID-19. Again, the results were very similar using this more restrictive definition but again with one exception: men aged 50–59 had an even more pronounced risk of severe COVID-19 compared to women of the same age (S1 Table).
In Table 3 we present the results from multivariate and conditional Cox regressions based on the cohort of opposite-sexed pairs of similar age living within the same household (n = 758,932 individuals). The magnitudes of men’s increased risk for hospitalization, ICU admission and death due to COVID-19 are the same among people included in the total cohort and the cohort of cohabiting persons; the estimates from standard Cox regressions based on the overall cohort (Table 2, Model 1 and 3) did not differ substantially from the corresponding analyses for the cohabiting cohort (Table 3, Model 1 and 2). Furthermore, adjusting for unobserved factors shared within households (Table 3, Model 3) and further adjustment for comorbidities and socioeconomic factors (Table 3, Model 4) in conditional Cox models did not have a substantial effect on the point estimates either.
In Table 4 we present the results from logistic regression models examining the association between sex and diagnosis of acute and severe secondary outcomes among hospitalized COVID-19 patients. After adjusting for age, men had higher odds of being diagnosed with viral pneumonia, acute respiratory distress syndrome, acute respiratory insufficiency, acute kidney injury, acute cardiac injury, sepsis, and cerebral infarcts. After adjusting for comorbidities and socioeconomic factors, the association of sex with acute cardiac injury and cerebral infarcts became insignificant as the point estimates dropped closer to one. In contrast, the association of sex with viral pneumonia, acute respiratory distress syndrome, acute respiratory insufficiency, acute kidney injury, and sepsis all remained significant, and the magnitude of the estimates remained essentially unchanged.
Discussion
In this large population-based study, we have observed a substantial male sex disadvantage in severe morbidity and mortality due to COVID-19. In general, men’s excess risk was most pronounced among middle aged people (50–59 years), in particular for the risk of COVID-19 related death. Adjusting for several important comorbidities and socioeconomic factors did not attenuate the observed associations. Further, adjusting for unobserved factors shared by individuals in the same household in a conditional Cox analysis did not provide any further explanation to the sex disparity in severe COVID-19 outcomes. In addition, among patients hospitalized due to COVID-19, men had an increased risk for complications associated with COVID-19 such as viral pneumonia, acute respiratory distress syndrome, acute respiratory insufficiency, acute kidney injury, and sepsis.
Our finding that men are at higher risk of severe COVID-19 infection and death compared to women, and that the increased relative risk varies across age-groups is consistent with previous studies.[1, 3, 19] The age-sex pattern of a pronounced higher relative risk for men around middle age has been hypothesized to be (at least partially) caused by the differential distribution of comorbidities across sexes and age strata. Women are generally reported to have a higher comorbidity burden, especially at older ages. However, women tend to suffer from more non-fatal chronic conditions such as migraine, depression, autoimmune and musculoskeletal diseases, whereas men have more life-threatening conditions such as cardiovascular diseases, hypertension, chronic lung diseases and type-2 diabetes,[28, 29] which are associated with a worse progression of COVID-19.[5] Thus, a higher burden of specific comorbidities in men may explain the markedly increased risk for severe COVID-19 outcomes around middle age, whereas a reduction of the increased relative risk for men at older ages may be explained by a survival effect leaving only the healthiest men to survive to old age. However, adjustments for comorbidities and socioeconomic factors did not attenuate the association between sex and severe COVID-19 outcomes in any age group. This finding is in line with previous studies on case fatality among patients with a confirmed infection reporting an increased risk among men independent of comorbidities, demographics, and health behaviors.[19, 30].
Gender-related differences in occupations, health-behaviors and attitudes have also been suggested to partly explain men’s increased risk for COVID-19 outcomes.[1] Studies have shown that women are more likely to perceive COVID-19 as a serious health problem and to agree and comply with restraining public policies.[6] However, such behaviors are primarily associated with risk for infection, whereas results from large meta-analyses and epidemiological surveillance demonstrate that COVID-19 infection rates are similar between sexes.[2, 4] Further, the test positivity rate among asymptomatic individuals has also been shown to be similar between sexes,[19] or even higher in women.[31] Therefore, the increased risk for morbidity and mortality in COVID-19 among men does not seem to be explained by higher risk of SARS-CoV-2 exposure or susceptibility. Our finding that among hospitalized patients with COVID-19, men had an increased risk for COVID-19 related complications (i.e., viral pneumonia, acute respiratory distress syndrome, acute respiratory insufficiency, acute kidney injury, and sepsis) independent of previous comorbidities and socioeconomic factors, also suggests that men have a higher risk of severe disease progression, rather than just increased virus exposure or susceptibility. It has also been suggested that gender-related social norms may cause men to postpone seeking out medical treatment, thereby experiencing more severe COVID-19 due to this delay. However, previous studies have found indications both for and against this tendency.[19, 32] Since we have not been able to adjust for these types of behaviors, we cannot rule out that delayed treatment among men partially explains our results. However, data from countries worldwide show that the sex disparity in COVID-19 case fatality and intensive therapy unit admissions is a global phenomenon with relatively homogenous relative risk estimates,[1, 4, 10] whereas gender differences in social behavior, life-style factors and comorbidities vary across countries. In addition, results from the within-household design provided no further explanation to the sex disparity, despite accounting for housing-related living conditions, including many social and lifestyle related factors to a large extent, as well as exposure to SARS-CoV-2, given that much of the transmission of SARS-CoV-2 occur within households.[33] Thus, the within-household design presumably partly accounts for gender-related differences, especially those who are related to risk of infection, even though gender-related differences in attitudes and behavior exist within households. Thus, we find it unlikely that gender differences in social behaviors is a driver of men’s increased risk in severe COVID-19 outcomes.
The observed sex disparity in COVID-19 outcomes could be a consequence of several biological sex differences, such as sex steroid hormone levels, sex chromosomes, differential genetic expression, and differences in the immune function, whereof some may interact with age.[10] Sex steroid hormones have known immunomodulatory functions; whereas estrogens may have both pro- and anti-inflammatory effects, androgens mainly act immunosuppressive.[34,35,36] Experimental animal studies support a beneficial immunomodulatory effect of estrogens towards coronaviruses,[37, 38] and epidemiological evidence indicates that hormone replacement treatment with estradiol may reduce COVID-19 mortality in post-menopausal women.[39] Although less studied, there are also some epidemiological indications of a potential negative effect of androgens on risk of COVID-19 infection, as a positive effect of anti-androgen treatment among male prostate cancer patients was observed.[40].
Women may also benefit from having two X-chromosomes that encode a large number of immune-related genes.[41, 42] As carriers of a single X-chromosome, men are more vulnerable for X-linked mutations, compared to women who are mosaic for X-linked genes. Due to random X-inactivation in females, there is usually no difference in dosage of X-linked gene products between sexes. However, incomplete inactivation of X-linked genes may occur at varying degree between genes and individuals,[43] and have been associated with higher prevalence of autoimmune diseases in women. For example, enhanced Toll-like receptor 7 (TLR7) gene expression has been associated with increased risk for SLE and other autoimmune disorders in women.[44] TLR7 is a pattern recognition receptor used to detect single stranded RNA, including coronaviruses. Ligand binding to TLR7 results in an increased production of type I interferons, which is an important mechanism of the innate immune system towards virus infections found to be stronger in women.[45, 46] In case studies, supposed loss-of-function mutations in TLR7 have been identified in young men with severe or fatal outcomes in COVID-19, without any known predisposing risk factors.[47, 48] A subsequent study screening for X-linked mutations in men, identified deleterious TLR7 variants in men with unexplained severe COVID-19, whereas none were identified among the mildly or asymptomatically infected men.[49] Angiotensin-converting enzyme 2 (ACE2) is the entry receptor for SARS-CoV-2 and the ACE2 gene is also coded on the X-chromosome and has been found to be regulated by estrogens.[50]ACE2 has a crucial role in the renin-angiotensin-aldosterone system (RAAS) which regulate blood pressure and electrolyte homeostasis. Thus, it has also been speculated that sex-derived differences in ACE2 expression and regulation may in part explain differences in COVID-19 outcomes.[51, 52].
Severe COVID-19 is associated with a state of dysregulated immune responses associated with excessive production of inflammatory cytokines and chemokines, inflammatory cell infiltration in the lungs, a so-called cytokine storm, causing lung injury and respiratory distress, together with lymphopenia. Deaths due to COVID-19 usually result from acute respiratory distress syndrome, acute respiratory failure, coagulopathy, organ failure and septic shock.[53] We found that men hospitalized due to COVID-19 were at higher risk of acute cardiac injury and cerebral infarcts compared to women, but this effect seemed to be confounded by underlying comorbidities. In contrast, we also found that male COVID-19 patients had a higher risk of developing pneumonia, acute respiratory distress syndrome, acute respiratory insufficiency, acute kidney injury, and sepsis compared to female patients, which seemed to be independent of underlying comorbidities and socioeconomic factors.
A major strength of this study is the total population-based setting with complete coverage of COVID-19 related hospitalizations and deaths, why this study is not subjected to selection bias. Another important strength is the use of administrative register data on socioeconomic factors. For example, these included information on housing arrangements that enabled the novel use of within-household comparisons to address unknown and unmeasured confounders shared by cohabitants in the study of sex disparities in severe COVID-19 outcomes. Further, the unique Stockholm Regional Healthcare Data Warehouse, which contains information on hospitalizations as well as data from primary and secondary care, improved our capability to identify individuals with important comorbidities, compared to using only hospital-based data.
There are also limitations to consider. This is an observational study, and as such we cannot determine the causal relationships that underlies the male sex disadvantage in morbidity and mortality due to COVID-19. This study could have been subjected to surveillance bias as men generally receive more specialist inpatient care, which could increase the chance of being detected with COVID-19 in health registers. To minimize this issue, we defined COVID-19 hospitalizations based on main diagnosis only. The accuracy of COVID-19 diagnoses in Swedish health and cause of death registers is presumably high but have not been formally validated. Sensitivity of hospital diagnoses as a measure of severe COVID-19 morbidity among older patients may be an issue since age discrimination in access to care has occurred.[54] Although it has not been shown that this age discrimination differs by sex, results of hospitalizations and ICU admissions among elderly should be interpreted more carefully. Some cases of comorbidity may have been misclassified. However, this is likely nondifferential with regard to sex. On the other hand, under-diagnosis, especially of less severe comorbidities, may differ between sexes and introduce bias. Residual confounding by life-style factors and comorbidities, especially mild cases not identified by registers, may be a limitation. However, these factors are likely captured by adjustments for socioeconomic factors and factors shared within households to some extent, though adjustments for these factors did not notably change the observed estimates. Emerging evidence seems to support an association between smoking and more severe COVID-19 outcomes.[55, 56] However, more women than men smoke daily in Sweden, although the difference is marginal.[57] Therefore, it is not likely that smoking status is a major driver of the observed sex disparity. Another consideration is that the follow-up period for hospitalizations and ICU admissions (ending on May 16, 2021) overlaps with the introduction of vaccination against SARS-CoV-2, which started in week 52, 2020. The vaccination coverage increased slowly during the first quarter of 2021 and thereafter started to increase more steeply. At the end of our study period, almost 10% had received two doses and about 30% had received at least one dose. Thus, vaccination status could potentially affect our results for hospitalizations and ICU admissions. However, results from our sensitivity analysis showed that HRs were the same during the first (before vaccination was initiated) and second wave (when the vaccination started) of the pandemic. Thus, it is unlikely that vaccination status interferes with our results. It is also unlikely that other factors that vary over time or between pandemic waves, such as stochastic transmission of the virus to different groups of people and new mutations and strain variants emerging, affect the sex ratio observed in our results.
Our results confirm that men have an excess risk of hospitalization, ICU admission and death due to COVID-19. This sex disparity varies across age groups and is most pronounced among middle aged men compared to women of the same age. Further, among hospitalized COVID-19 patients, men are more likely to be diagnosed with COVID-19 related complications. Different explanations have been suggested to explain men’s vulnerability to COVID-19, including gender related health behaviors, differential distribution of comorbidities and biological sex differences such as sex steroid hormones, genetic constitution, or differential gene expression. The reason for men’s excessive risk is likely multifactorial with a complex interplay between several factors. In this study we adjusted for multiple confounders, including important comorbidities, socioeconomic factors as well as unmeasured household-related factors. These adjustments had no substantial impact on men’s excess risk of severe COVID-19 outcomes. Thus, our results motivate a focus in future studies on biological mechanisms that influence the immune response towards SARS-CoV-2 to help explain women’s advantage in COVID-19. This study also highlights the importance to consider the combined effect of age and sex when defining those at highest risk of severe COVID-19 outcomes.
References
Gebhard C, et al. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29.
Global Health 5050. The sex gender and covid-19 project. n.d. October 7, 2021]; Available from: https://globalhealth5050.org/the-sex-gender-and-covid-19-project/.
Ahrenfeldt LJ, et al. Sex and age differences in COVID-19 mortality in Europe. Wien Klin Wochenschr. 2021;133(7–8):393–8.
Peckham H, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317.
Williamson EJ, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6.
Galasso V, et al. Gender differences in COVID-19 attitudes and behavior: Panel evidence from eight countries. Proc Natl Acad Sci U S A. 2020;117(44):27285–91.
Barber SJ, Kim H. COVID-19 Worries and Behavior Changes in Older and Younger Men and Women. J Gerontol B Psychol Sci Soc Sci. 2021;76(2):e17–23.
Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38.
Takahashi T, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–20.
Scully EP, et al. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442–7.
Kadel S, Kovats S. Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Front Immunol. 2018;9:1653.
Butterworth M, McClellan B, Allansmith M. Influence of sex in immunoglobulin levels. Nature. 1967;214(5094):1224–5.
Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159(3):229–31.
Alghamdi IG, et al. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med. 2014;7:417–23.
Flanagan KL, et al. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu Rev Cell Dev Biol. 2017;33:577–99.
Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):9–15.
Fischinger S, et al. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41(2):239–49.
Fathi A, Addo MM, Dahlke C. Sex Differences in Immunity: Implications for the Development of Novel Vaccines Against Emerging Pathogens. Front Immunol. 2020;11:601170.
Scully EP, et al. Sex and Gender Differences in Testing, Hospital Admission, Clinical Presentation, and Drivers of Severe Outcomes From COVID-19. Open Forum Infect Dis. 2021;8(9):ofab448.
Del Valle DM, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–43.
Ludvigsson JF, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–67.
Wändell P, et al. Most common diseases diagnosed in primary care in Stockholm, Sweden, in 2011. Fam Pract. 2013;30(5):506–13.
The Swedish Intensive Care Registry (SIR). 2021; Available from: https://www.icuregswe.org/en/.
Emilsson L, et al. Review of 103 Swedish Healthcare Quality Registries. J Intern Med. 2015;277(1):94–136.
Brooke HL, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–73.
Ludvigsson JF, et al. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34(4):423–37.
Ahlberg M, et al. Association of SARS-CoV-2 Test Status and Pregnancy Outcomes. JAMA. 2020;324(17):1782–5.
Ahrenfeldt LJ, et al. Sex Differences in Comorbidity and Frailty in Europe. Int J Public Health. 2019;64(7):1025–36.
Case A, Paxson C. Sex differences in morbidity and mortality. Demography. 2005;42(2):189–214.
Alkhouli M, et al. Sex Differences in Case Fatality Rate of COVID-19: Insights From a Multinational Registry. Mayo Clin Proc. 2020;95(8):1613–20.
Kalish H, et al., Undiagnosed SARS-CoV-2 seropositivity during the first 6 months of the COVID-19 pandemic in the United States. Sci Transl Med, 2021. 13(601).
Cobre AF, et al. Risk factors associated with delay in diagnosis and mortality in patients with COVID-19 in the city of Rio de Janeiro, Brazil. Cien Saude Colet. 2020;25(suppl 2):4131–40.
Madewell ZJ, et al. Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3(12):e2031756.
Phiel KL, et al. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97(1):107–13.
Taneja V, Sex Hormones Determine Immune Response. Front Immunol, 2018. 9: p. 1931.
Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–74.
Channappanavar R, et al. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. J Immunol. 2017;198(10):4046–53.
Rambhatla A, et al. COVID-19 Infection in Men on Testosterone Replacement Therapy. J Sex Med. 2021;18(1):215–8.
Seeland U, et al. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med. 2020;18(1):369.
Montopoli M, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol. 2020;31(8):1040–5.
Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10(8):594–604.
Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737–44.
Tukiainen T, et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550(7675):244–8.
Souyris M, et al., TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol, 2018. 3(19).
Berghöfer B, et al. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. 2006;177(4):2088–96.
Webb K, et al. Sex and Pubertal Differences in the Type 1 Interferon Pathway Associate With Both X Chromosome Number and Serum Sex Hormone Concentration. Front Immunol. 2018;9:3167.
van der Made CI, et al. Presence of Genetic Variants Among Young Men With Severe COVID-19. JAMA. 2020;324(7):1–11.
Solanich X, et al. Genetic Screening for TLR7 Variants in Young and Previously Healthy Men With Severe COVID-19. Front Immunol. 2021;12:719115.
Asano T, et al., X-linked recessive TLR7 deficiency in ~ 1% of men under 60 years old with life-threatening COVID-19. Sci Immunol, 2021. 6(62).
Liu J, et al. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ. 2010;1(1):6.
Haitao T, et al. COVID-19 and Sex Differences: Mechanisms and Biomarkers. Mayo Clin Proc. 2020;95(10):2189–203.
Bourgonje AR, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251(3):228–48.
Wu C, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–43.
K OC, et al., Older Persons and the Right to Health in the Nordics during COVID-19. Eur J Health Law, 2021: p. 1–28.
Patanavanich R, et al. Active smokers are at higher risk of COVID-19 death: A systematic review and meta-analysis. Nicotine Tob Res; 2022.
Clift AK, et al. Smoking and COVID-19 outcomes: an observational and Mendelian randomisation study using the UK Biobank cohort. Thorax. 2022;77(1):65–73.
Public Health Agency of Sweden [Folkhälsomyndigheten]. Tobaksrökning, daglig [Internet]. 2022 2022-08-29]; Available from: https://www.folkhalsomyndigheten.se/fu-tobaksrokning.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was approved by the regional ethical review board in Umeå, Sweden (Dnr: 2020–03122).
Informed consent
Informed consent was not obtained as this was a register study and all information was anonymized.
Consent to publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sieurin, J., Brandén, G., Magnusson, C. et al. A population-based cohort study of sex and risk of severe outcomes in covid-19. Eur J Epidemiol 37, 1159–1169 (2022). https://doi.org/10.1007/s10654-022-00919-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-022-00919-9