Abstract
Arsenite (As(III)) was considered to be of great concern in acid mine drainage (AMD). A promising approach for cleaning up of arsenite from AMD is microbial oxidation of As(III) followed by adsorptions. However, there is virtually no research about the acidophilic bioreactor for As(III) oxidation so far. In this study, we formed a new biofilm bioreactor with a consortium of acidophilic As(III) oxidation bacteria. It is totally chemoautotrophic, with no need to add any carbon or other materials during the operations. It works well under pH 3.0–4.0, capable of oxidizing 1.0–20.0 mg/L As(III) in 3.0–4.5 h, respectively. A continuous operation of the bioreactor suggests that it is very stable and sustainable. Functional gene detection indicated that the biofilms possessed a unique diversity of As(III) oxidase genes. Taken together, this acidophilic bioreactor has great potential for industrial applications in the cleaning up of As(III) from AMD solution.
Similar content being viewed by others
Introduction
Acid mine drainage (AMD) derived from abandoned and active mines is the formation and flow of polluted water that contains high levels of sulfuric acid and heavy metal/metalloids, including Fe, Mn, Alu, Zn, Pb, Cd and As (Akcil, Koldas 2006; Chen et al. 2014; Gutiérrez et al., 2016). The highly acidic stream of AMD was formed through microbial and geochemical reactions between surface water and sulfur-bearing minerals. The generated yellow fluid further dissolves heavy metals/metalloids-bearing minerals, leading to severe contaminations of groundwater, surface water, sediments and soils (Zhang et al. 2019; Hierro et al. 2014; Anawar. 2015; Coudert et al. 2019). Therefore, AMD is one of the most severe environmental threats, and the development of sustainable and environment-friendly approaches for the treatment of AMD are urgently required.
Among all the metals/metalloids existing in AMD, arsenic (As) was generally considered to be of great concern (Johnson, Hallberg 2005). Because As is always closely associated with sulfur ore mineralization, AMD often contains high contents of As, of which dominant form is As(III). For example, the AMDs derived from the abandoned Onyeama Coal Mine contained 9.9–39.6 mg/L soluble As(III) (Ozoko 2015). It was shown that As(III) is much more mobile and approximately 60 times more toxic than As(V). As(III) is highly toxic to majority of all the organisms, including plants, animal and microorganisms. It ranks as the first class of human carcinogen as reported by the World Health Organization. Additionally, a geochemical survey indicated that when the fluid from AMD flowed out and mixed with the soils in estuarine, ~60–100% of dissolved Fe, Alu, Mn, Pb, Zn, Co and Cu were immobilized by adsorption and precipitation; however, almost all As remained as soluble forms (Cheng et al. 2009). This suggests that As is highly mobile during the flow of AMD, and very dangerous to the environment. If AMD flows into surface waterways, mobile arsenic may result in severe environmental issues over a large area. Therefore, removal of As(III) from AMD should be given priority.
A variety of alternative approaches were used to remediate AMD (Park et al. 2019; Naidu et al. 2019). Majority of these methods involved in attempts to enhance the pH values of the solution, inhibit the oxidation of minerals caused by dissolved oxygen and microorganisms, and precipitate/co-precipitate and coagulate dissolved heavy metals and metalloids in AMD (Coudert et al. 2019). Although these efforts achieved some results for the AMD remediation, their effectivity and sustainability remain to be questionable due to highly complicated and changeable geochemical conditions, mineralization types, microbial activities and local climate in AMD areas (Naidu et al. 2019). It is estimated that when anaerobic bioremediation methods were used for the treatment of AMD, the dissimilatory As(V)-respiratory prokaryotes (DARPs)-simulated reductive mobilization of As-bearing minerals would be greatly increased; moreover, rises in pH values of AMD often lead to higher solubility of arsenic (Hierro et al. 2014; Bondu et al. 2017; Anawar. 2015; Chen et al. 2020). This suggests that current AMD treatment methods were not capable of cleaning up arsenic from AMD.
Recently, a consortium of sulfate-reducing bacteria was used to treat arsenic-rich AMD solution (Sun et al. 2019). Through the microbial reaction, sulfate was reduced to sulfide, coupled with the oxidation of glycerol to acetate or CO2. The formed sulfide reacts with arsenite, producing precipitations of As2S3 and AsS. Although 100% As was finally precipitated from AMD after 94 days of anaerobic incubations, time consumption and organic carbon requirement would hamper the practical application of this technique (Sun et al. 2019).
Microbial oxidation of As(III) by biofilm bioreactors followed by adsorption is a promising and environment-friendly technique for the treatment of As(III)-contaminated groundwater and surface water (Sun et al. 2011; Li et al. 2016). However, there is virtually no research about the construction of acidophilic biofilm bioreactor so far. In this study, we constructed a novel biofilm bioreactor using a population of acidophilic As(III)-oxidizing bacteria enriched from the liquid of an AMD from the abandoned Shimen Realgar Mine. This biofilm bioreactor combined with the use of an As(V) sorbent is capable of completely removing all As from AMD liquids. It is chemoautotrophic, efficient, and very stable, and thus has great potential for industrial applications.
Materials and methods
Enrichment of acidophilic arsenite-oxidizing bacteria
Slurry samples with a pH of 3.5 were collected from an acid mine drainage (AMD) in the Shimen Realgar Mine. The As(III)-oxidizing activity of the slurries was examined by the microcosm assay as described elsewhere (Zeng et al. 2016). Briefly, ~5.0 g of samples was mixed with 95 mL of minimal mineral salts (MMS) medium (adjusted to pH 3.5) (Wang et al. 2017) supplemented with 5.0 mM As(III) and 5.0 mM lactate as the sole carbon source. The flasks were incubated under aerobic conditions with continuous shaking at a speed of 150 rpm. At intervals of 1–5 days, ~1.0 mL of mixtures was removed from the flask for the examination of arsenic species using high performance liquid chromatography coupled with inductively coupled plasma mass spectrometry technique (Wang et al. 2017).
Microbial enrichment technique was used to isolate a consortium of acidophilic As(III)-oxidizing bacteria (AOB) from the AMD slurries (Yang et al. 2017). A modified synthetic AMD solution was used for the enrichment, which consists of 0.24 g CaSO4·2H2O, 0.63 g CdSO4·3H2O, 0.71 g HgSO4, 0.73 g PbSO4, 1135.00 g FeSO4·7H2O, 33.50 g K2SO4, 75.25 g MnSO4·H2O, 49.45 g MgSO4, 43.55 g Na2SO4, 130.40 g NiSO4·6H2O and 9.45 g ZnSO4·7H2O, amended with different concentrations of As(III), and added distilled water to 500 mL (Hwang, Jho 2018). The solution was adjusted to a pH of 3.5. Approximately 40.0 g of AMD slurries were mixed with 160.0 mL of the synthetic AMD solution amended with 10.0 mM NaHCO3 and 5.0 mM As(III). The mixtures were incubated under aerobic conditions at room temperature until all As(III) was oxidized in to As(V). Second round of enrichment cultivation was performed by mixing 40.0 mL of the cultures with 160.0 mL fresh AMD solution amended with 10.0 mM NaHCO3 and 5.0 mM As(III). The mixtures were incubated under aerobic conditions until all As(III) was oxidized. After 5 rounds of enrichments, the bacterial cells were collected by centrifugation. The microbial precipitations were suspended with 10.0 mL of the synthetic AMD solution and the suspended bacterial cells were temporarily stored at 4 °C for use.
Construction and characterization of a novel acidophilic biofilm bioreactor
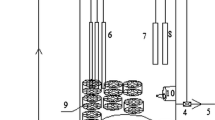
As shown in Fig. 1, the biofilm bioreactor consists of a container with a volume of 1000 mL, a compressed air provider and a peristaltic pump for mixing the solution. Perlite particles were used as the biofilm carrier for bioreactor construction. Perlites were placed into the container and saturated with the synthetic AMD solution. After 2.0 days, the old AMD solution was replaced with a fresh one amended with 5.0 mM As(III) and 1.0% yeast extract. Approximately 10 mL of the enriched cultures was added into the perlite suspensions, and mixed well by stirring. The container was supplied with compressed air occasionally, and incubated at room temperature. At intervals of 5.0 days, a half of the AMD solution in the container was replaced with the fresh one amended with 5.0 mM As(III) and 1.0% yeast extract. After 50 days, a piece of perlites was removed from the container for determining whether any biofilms were formed using the Scanning Electron Microscope (SEM) technique as described elsewhere (Li et al. 2016). If dense biofilms were formed on the perlites, the AMD solution in the container was replaced with the fresh one amended with 5.0 mM As(III) and 10.0 mM NaHCO3 for selection of chemo-autotrophic AOB. At intervals of 5 days, the solution in the bioreactor container was replaced with the fresh one. After 1 month of acclimatization, the bioreactor was ready for operations.
Operations of the acidophilic biofilm bioreactor under different conditions
Operations of the acidophilic biofilm bioreactor were conducted using the synthetic AMD solution adjusted to a different pH, and amended with different concentrations of As(III). To examine the effects of pH on the As(III)-oxidizing activity of the bioreactor, the bioreactor was operated with the synthetic AMD solution adjusted to a pH of 2.5, 3.0, 3.5, 4.0 and 4.5, respectively, and amended with 20.0 mg/L As(III). To determine the effects of initial As(III) concentrations on the As(III)-oxidizing activity of the bioreactor, the bioreactor was operated with the synthetic AMD (pH 3.5) amended with 1.0, 5.0, 10.0, 15.0 or 20.0 mg/L As(III), respectively. To determine an optimal hydraulic retention time (HRT) for bioreactor operations, the bioreactor was loaded with the synthetic AMD solution (pH 3.5) amended with 20.0 mg/L As(III), and operated under standard conditions. To detect the stability of the bioreactor, the bioreactor was operated one time per day, and the time required for complete oxidation of 20.0 mg/L As(III) was determined every 2 days; after 30 days of operations, if the time required for complete oxidation of all As(III) decreased by <10%, the bioreactor was considered to be “stable”. For all the operations of the acidophilic bioreactor, at intervals of 1–5 days, ~0.8 mL of treated AMD solutions were removed from the tubes for measuring the As(III) and As(V) concentrations.
Identification of As(III) oxidase genes from the biofilms of the bioreactor
The microbial gene encoding the AioA unit of As(III) oxidase was used as a genetic marker for examination of the existence of AOB. A pair of aioA gene-specific primers was designed based on the known aioA gene sequences of bacteria deposited in GenBank (Chen et al. 2020). The primers were synthesized by TaKaRa Company (TaKaRa Biotechnology, Japan). The bacterial cells-bearing perlites were ground into fine particles for extraction of microbial genomic DNA using Presto™ Soil DNA Extraction Kit. PCR reactions were set up and carried out as described elsewhere (Zeng et al. 2016). Amplified DNA fragments were used for construction of DNA library that was further sequenced and analyzed using BLAST online sever (Zeng et al. 2018). A phylogenetic tree was constructed based on the multiple sequence alignment of the obtained AioAs and their closely related known AioA proteins from GenBank database (Shi et al. 2018; Zhu et al. 2019).
Analysis of microbial community structure of the biofilms
Biofilm genomic DNA was extracted using Presto™ Soil DNA Extraction Kit. Bacterial 16S rRNA gene′s V4 and V5 regions-specific barcoded primers were used to prepare amplicons using Trans StartFast pfu DNA Polymerase (Sun et al. 2018; Ngegla et al. 2020). DNA sequences were analyzed on ABI GeneAmp® 9700. Raw sequences were merged with FLASH. Adaptors and low-quality sequences were removed with Trimmomatic. Chimeras were removed using UCHIME (Chen et al. 2017, 2020). High-quality DNA sequences were clustered into OTUs using UCLUST version 1.2.22. Microbial composition was further analyzed and compared with others using QIIME and UPGMA (Edwardson, Hollibaugh 2017).
Results
As(III)-oxidizing activity of the AMD slurries
The pH value of the natural AMD water from the abandoned Shimen Realgar Mine was 3.5. We collected the slurries of AMD sediments from the depth of 20 cm. The As(III) oxidation activity of the AMD slurries was detected by mixing 5.0 g slurry samples with 95.0 mL of the MMS medium adjusted to a pH of 3.5. The suspensions were amended with 5.0 mM As(III), and 5.0 mM lactate. After 16.0 h of cultivation under aerobic conditions, all As(III) was converted to As(V) (Fig. 2). This indicated that the microbial community in the slurries collected from the acid mine drainage had significant As(III)-oxidizing activities under a pH of 3.5, and there were some acidophilic As(III)-oxidizing bacteria in the AMD slurries. The concentration of the total As in the microcosm slightly decreased during the microbial reactions (Fig. 2); this should be due to that As(V) is much easier to be adsorbed than As(III) by the soils (Fig. 2).
Construction and characterization of an acidophilic biofilm bioreactor
A consortium of acidophilic As(III)-oxidizing bacteria were obtained by 5 rounds of enrichment cultivation from the slurries collected from an AMD in the abandoned Shimen Realgar Mine. A novel biofilm bioreactor was constructed as shown in Fig. 1 with the enriched cultures using perlites as the biofilm carries. Approximately 10.0 mL of the enriched cultures were inoculated to the perlite particles that were saturated with the synthetic AMD water (pH 3.5) amended with 5.0 mM As(III) and 1.0% yeast extract. After 50 days of incubation with periodic replacement of the AMD solution with the fresh one, a SEM observation demonstrated that there were dense and thick biofilms cross-linked by extracellular polymeric substances (EPS)-like materials adhered on the perlite particles; however, the control perlites had very smooth surfaces (Fig. 3a). This suggests that biofilms of acidophilic AOB were successfully formed on the surfaces of perlite particles. The biofilms were further acclimatized with the synthetic AMD solution amended with 5.0 mM As(III) and 10.0 mM NaHCO3 for excluding heterotrophic AOB from the biofilms.
Construction and characterization of the acidophilic biofilm bioreactor for As(III) oxidation. a Morphology of the acidophilic biofilms derived from a consortium of As(III)-oxidizing bacteria from an AMD (pH 3.5) in the abandoned Shimen Realgar Mine. b Operations of the acidophilic biofilm bioreactor under different pH values. The initial As(III) concentration in the synthetic AMD solution was 20.0 mg/L. c Operations of the acidophilic biofilm bioreactor at different initial concentrations of As(III) dissolved in the synthetic AMD solution (pH of 3.5). d Long-term operations of the acidophilic biofilm bioreactor for As(III) oxidation. The initial concentration of As(III) was 20.0 mg/L. The As(III)-oxidizing activity was expressed as the minimal time required for complete oxidation of all As(III) in the synthetic AMD solution
To examine how the pH values of the AMD solution affect the As(III)-oxidizing activity of the acidophilic biofilm bioreactor, we operated the bioreactor by treating synthetic AMD solution containing 20.0 mg/L As(III), adjusted a pH value of 2.5, 3.0, 3.5, 4.0 and 4.5, respectively. Figure 3b shows the As(III)-oxidizing activity of the bioreactor under different pH values, which was expressed as the time needed for complete oxidation of 20.0 mg/L As(III). We found that ~23.3, 8.3, 4.6, 6.3, 20.8 h were needed to completely oxidize all the loaded As(III) into As(V) under pH 2.5, 3.0, 3.5, 4.0 and 4.5, respectively (Fig. 3b). This suggests that this bioreactor has efficient As(III)-oxidizing activities under a pH of 3.0-4.0, and the optimal pH is 3.5. Lower or higher pH values of the AMD water would lead a significant decline of the As(III)-oxidizing activity.
Figure 3c shows the As(III)-oxidizing activity of the acidophilic biofilm bioreactor at different initial concentrations of As(III), which were dissolved in the synthetic AMD solution adjusted to a pH of 3.5. We found that ~3.0, 3.5, 3.8, 4.2 and 4.5 h were needed to completely oxidize all loaded 1.0, 5.0, 10.0, 15.0 and 20.0 mg/L As(III) into As(V), respectively (Fig. 3c). This suggests that the bioreactor can efficiently oxidize 1.0–20.0 mg/L As(III) in the synthetic AMD solution. We continuously operated the bioreactor at an initial As(III) concentration of 20.0 mg/L for more than 30 days, and found that the As(III)-oxidizing activity decreased by <5%; after 2 years of discontinuous operations, the As(III)-oxidizing activity of the bioreactor decreased by only 7.0% (Fig. 3d).
Analysis of the bacterial As(III) oxidase genes from acidophilic biofilms of the bioreactor
A piece of biofilms-bearing perlite particles were collected from the bioreactor for extraction of the bacterial genomic DNA and further cloning of the bacterial genes encoding the AioA subunit of As(III) oxidase. As shown in Fig. 4, a total of 18 novel AioA proteins were identified from the biofilms. They were referred to as AcidoA1-18. BLAST searches and sequence comparisons indicated that the obtained acidoA1-18 sequences showed 47.1–98.0% identities to the known AioA proteins deposited in the GenBank database, suggesting that the AioA proteins of acidophilic As(III)-oxidizing bacteria are similar to those of general As(III)-oxidizing bacteria. A phylogenetic tree was constructed using the obtained AioA protein sequences from this study and their closely related known AioA sequences from GenBank (Fig. 4). A known AioA from Thermus aquaticus was included as an outgroup. The tree showed that all acidoA1-18 proteins can be grouped into four distinct clusters: cluster 1 (AcidoA1), showing 47% and 49% identities with the AioAs from Aliihoeflea sp. 2WW and Kaistia sp. SCN65-12, respectively; cluster 2 (AcidoA2, AcidoA3, AcidoA4, AcidoA5, AcidoA6, AcidoA7 and AcidoA8), showing 78-92% identities to the AioAs from Herminiimonas arsenicoxydans and Herbaspirillum chlorophenolicum, respectively; cluster 3 (AciodoA9, AcidoA10, AcidoA11 and AcidoA12), showing 80–94% identities with the AioAs from Achromobacter arsenitoxydans, Alcaligenes faecalis, Paraburkholderia ginsengisoli, Burkholderia arationisi and Cupriavidus necator, respectively; cluster 4 (AcidoA13), showing 81–92% identities with the AioAs from Paraburkholderia ginsengisoli, Burkholderia arationisi and Cupriavidus necator; cluster 5 (AcidoA13, AcidoA14, AcidoA15, AcidoA16, AcidoA17 and AcidoA18), showing 76–98% identities with the AioA from Pandoraea pnomenusa, respectively. All these known AioA-bearing bacteria are affiliated to α-Proteobacteria; this suggests that all the AcidoA1-18 proteins from the acidophilic As(III)-oxidizing bacteria belong to the AioA protein family affiliated to the phylum α-Proteobacteria.
Microbial compositions of the acidophilic biofilm bioreactor
The microbial composition of the biofilms in the bioreactor was analyzed by high-throughput sequencing technique. As shown in Fig. 5, we found the dominant bacteria in phylum level in the biofilms include Proteobacteria (66.5% of the total microbial community in the biofilm bioreactor), Sacchairibacteria (16.3%), Actinabacteria (8.4%), Bacteroidetes (5.1%) and Firmicures (2.9%); others (0.8%) are less dominant. This microbial composition is significantly different from that of the non-acidophilic biofilm bioreactor for As(III) oxidation, of which the most dominant bacterial phylum is Bacteroidetes (44.2%) (Li et al. 2016).
Discussions
Environmental implications
Acid mine drainage is a naturally occurring highly acidic metals-rich water flow generated by biological and chemical bleaching of natural sulfide-bearing minerals in the presence of water and oxygen (Akcil, Koldas 2006; Chen et al. 2016). Because AMD solution is highly acidic and contains various heavy metals and metalloids, such as Pb, As, Hg, Cd, Fe and Cu, it is highly toxic to the aquatic and soil ecosystems. AMD is considered to be one of the major threats for aquatic ecosystems and soils (Grande et al. 2018; Moreno-González et al. 2020). Therefore, the AMD solution must be cleaned up or remediated before it flows away from the mines/tailings.
Many approaches were used to treat the AMD solution, such as neutralization with lime, adsorptions with ion exchange resins, precipitations with different materials, and treatment by wetlands and sulfate-reducing bacteria, as well as passive bioremediation (Akcil, Koldas 2006; Machodi and Daramola 2019; Liu et al. 2015; Wang et al. 2019; Sun et al. 2019; Fernandez-Rojo et al. 2019). However, most these methods were either non-sustainable or expensive (Naidu et al. 2019; Park et al. 2019). Moreover, the AMD solution contains high concentrations of As(III). As the AMD water flows through soils, the pH of the solution would be increased, leading to precipitations of most heavy metals/metalloids; however, the mobilization of As significantly often enhances as the pH of AMD solution rises (Bondu et al. 2017; Anawar et al., 2015). This strongly suggests that more feasible methods are needed to clean up As(III) from the As(III)-containing AMD solution.
Microbial oxidation of As(III) combined with subsequent adsorptions of As(V) was proved to be a promising environment-friendly approach for the treatment of As(III)-contaminated water (Li et al. 2016). However, the extremely acidic conditions markedly blocked the proliferation of As(III)-oxidizing bacteria in the AMD solution. In addition, little is known about the diversity, activity and biofilm formation of acidophilic As(III)-oxidizing bacteria from any AMD solution so far (Villegas-Plazas et al., 2019). This limited the construction of acidophilic biofilm bioreactors. In this study, we found that the semimetal slurries collected from an AMD had significant As(III) oxidation activity under a pH of 3.5. Using the enriched cultures of the slurries, we successfully constructed an acidophilic biofilm bioreactor. Operations of this bioreactor under different conditions indicated that it is chemoautotrophic, efficient, stable and environment-friendly, and thus has great potential for industrial applications.
The features of the acidophilic AOB biofilms
We found that it is hard for acidophilic AOB to form biofilms. Compared to the general autotrophic AOB, acidophilic AOB needed much longer time and required organic carbon as their carbon source for biofilm formations. Moreover, as shown in Fig. S1, the SEM observations indicated that the biofilm morphology of acidophilic AOB significantly differs from that of general AOB. The latter looks like a lot of biomass cross-linked by many EPS-like materials (Li et al. 2016), while the former looks like a biomass stack with a few of EPS-like materials. We also observed that there were some ribbon materials attached on the acidophilic AOB biofilms, suggesting a unique structure of the acidophilic biofilms.
We found that the optimal pH for the bioreactor operations is between 3.0 and 4.0, and higher or lower pH values would lead to a significant decrease of the As(III)-oxidizing activity. This suggests that acidophilic AOB are able to function under only a narrow pH range.
We compared the compositions of the microbial communities from the tailing of Shimen Realgar Mines (A) (Zeng et al. 2016) with those from the bioreactor constructed with a general AOB consortium from the same tailing (B) (Li et al. 2016) and those from the bioreactor constructed with an acidophilic AOB consortium from the same tailing (C, this study). We found that their microbial community structures significantly differ from each other. Their microbial diversity order from higher to lower is: A > B > C. This suggests that extreme or restricted conditions would significantly reduce the microbial community diversity. It is interesting to see that the most predominant bacteria from the acidophilic bioreactor (C) is Proteobacteria (66.5% of the total microbial community), which is also the most dominant in the tailing; in comparison, the most dominant one in the non-acidophilic bioreactor (B) is Bacteroidetes (44.2%), which is only the third most dominant bacteria in the tailing (Zeng et al. 2016). This observation suggests that the acidophilic and non-acidophilic AOB are largely affiliated to two distinct groups of bacteria from the tailing area.
We identified a total of 18 novel AioAs of As(III) oxidase from the acidophilic bioreactor, of which amino acid sequences are significantly different from those of the AioAs from the non-acidophilic bioreactor. All the AioA proteins from the acidophilic biofilm bioreactor are affiliated to the AioA family of α-Proteobacteria (Fig. 4), whereas those from the non-acidophilic bioreactor are affiliated to the AioA families of α-Proteobacteria and β-Proteobacteria. This suggests that the AioA proteins from acidophilic AOB may have lower diversity and undergo restricted evolution compared to the AioAs from non-acidophilic AOB.
Stability and sustainability of the acidophilic biofilm bioreactor
Although it is very hard and needs much longer time for the acidophilic AOB to form biofilms, the formed acidophilic biofilms were proved to be very stable as revealed by long-term operations. We continuously operated the bioreactor at an initial As(III) concentration of 20.0 mg/L for more than 30 days, and found that the As(III)-oxidizing activity decreased by only less than 5%. This indicated that the stability of the acidophilic biofilm bioreactor is comparable to that of the non-acidophilic biofilm bioreactor (Li et al. 2016).
Chemoautotrophic acidophilic AOB grow very slowly in the presence of NaHCO3 as the sole carbon source. We thus constructed the biofilm bioreactor using 1.0% yeast extract as the sole carbon source. After the biofilms were formed, we replaced the yeast extract with NaHCO3 as the sole carbon source; this would directionally select the autotrophic AOB and excluded the heterotrophic AOB from the generated biofilms. The acclimatized biofilms are thus totally autotrophic. During the operations of the bioreactor, no any carbon source is required, and the naturally dissolved CO2 in the water is enough for the slow replacement of the dead cells in the biofilms. We found that after 2 years of discontinuous operations, the As(III)-oxidizing activity of the bioreactor decreased by only 7.0% (Fig. 3d). These observations indicated that this acidophilic bioreactor is autotrophic and very stable, and thus is sustainable.
Conclusions
As(III) was considered to be a great concern in AMD solution. However, there is lack of feasible and environment-friendly approaches for removal of As(III) from the AMD solution. In this study, we constructed a novel biofilm bioreactor using a consortium of acidophilic bacteria enriched from an AMD solution. This bioreactor was capable of efficiently oxidizing As(III) dissolved in the synthetic AMD solution under a pH of 2.5–4.5. The optimal pH for operation was between 3.0 and 4.0. Long-term operations suggested that the bioreactor was very stable. More important, the bioreactor is totally autotrophic, with no need to add any carbon source and other substances during the operations. The dissolved CO2 was the only carbon source for maintaining the microbial metabolism and regeneration. A diversity of novel aioA genes were identified from the acidophilic biofilms. We also found that the diversity of the microbial community in the acidophilic biofilms are unique. These data suggest that this acidophilic biofilm bioreactor has high As(III)-oxidizing activities under pH values of 3.0–4.0, and is very stable, sustainable and environment-friendly, and thus has a great potential for industrial applications in the cleaning up of As(III) from naturally occurring AMD solution.
References
Akcil A, Koldas S (2006) Acid mine drainage (AMD): causes, treatment, and case studies. J Clean Prod 14:1139–1145
Anawar HM (2015) Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction and climate knowledge. J Environ Manage 158:111–121
Bondu R, Cloutier V, Rosa E, Benzaazoua M (2017) Mobility and speciation of geogenic arsenic in bedrock groundwater from the Canadian Shield in western Quebec, Canada. Sci Total Environ 2017574:509–519
Chen LX, Huang LN, Méndez-García C, Kuang JL, Hua ZS, Liu J, Shu WS (2016) Microbial community, processes and functions in acid mine drainage ecosystems. Curr Opin Biotechnol 38:150–158
Chen X, Zeng XC, Kawa YK, Wu W, Zhu X, Ullah Z, Wang Y (2020) Microbial reactions and environmental factors affecting the dissolution and release of arsenic in the severely contaminated soils under anaerobic or aerobic conditions. Ecotoxicol Environ Saf 189:109946
Chen X, Zeng XC, Wang J, Deng Y, Ma T,E,G, Mu Y, Yang Y, Li H, Wang Y (2017) Microbial communities involved in arsenic mobilization and release from the deep sediments into groundwater in Jianghan plain, Central China. Sci Total Environ 579:989–999
Chen YT, Li JT, Chen LX, Hua ZH, Huang LN, Liu J, Xu BB, Liao B, Shu WS (2014) Biogeochemical processes governing natural pyrite oxidation and release of acid metalliferous drainage. Environ Sci Technol 48:5537–5545
Cheng H, Hu Y, Luo J, Xu B, Zhao J (2009) Geochemical processes controlling fate and transport of arsenic in acid mine drainage (AMD) and natural systems. J Hazard Mater 165:13–26
Coudert L, Bondu R, Rakotonimaro TV, Rosa E, Guittonny M, Neculita CM (2019) Treatment of As-rich mine effluents and produced residues stability: current knowledge and research priorities for gold mining. J Hazard Mater 386:121920
Edwardson CF, Hollibaugh JT (2017) Metatranscriptomic analysis of prokaryotic communities active in sulfur and arsenic cycling in Mono Lake, California, USA. ISME J 11:2195–2208
Fernandez-Rojo L, Casiot C, Laroche E, Tardy V, Bruneel O, Delpoux S, Desoeuvre A, Grapin G, Savignac J, Boisson J, Morin G, Battaglia-Brunet F, Joulian C, Héry M (2019) A field-pilot for passive bioremediation of As-rich acid mine drainage. J Environ Manage 232:910–918
Grande JA, Santisteban M, de la Torre ML, Dávila JM, Pérez-Ostalé E (2018) Map of impact by acid mine drainage in the river network of The Iberian Pyrite Belt (Sw Spain). Chemosphere 199:269–277
Gutiérrez M, Mickus K, Camacho LM (2016) Abandoned Pb Zn mining wastes and their mobility as proxy to toxicity: A review. Sci Total Environ 565:392–400
Hierro A1, Olías M, Ketterer ME, Vaca F, Borrego J, Cánovas CR, Bolivar JP (2014) Geochemical behavior of metals and metalloids in an estuary affected by acid mine drainage (AMD). Environ Sci Pollut Res Int 21:2611–2627
Hwang SK, Jho EH (2018) Heavy metal and sulfate removal from sulfate-rich synthetic mine drainages using sulfate reducing bacteria. Sci Total Environ 635:1308–1316
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14
Li H, Zeng XC, He Z, Chen X, E G, Han Y, Wang Y (2016) Long-term performance of rapid oxidation of arsenite in simulated groundwater using a population of arsenite-oxidizing microorganisms in a bioreactor. Water Res 101:393–401
Liu F, Zhou J, Zhou L, Zhang S, Liu L, Wang M (2015) Effect of neutralized solid waste generated in lime neutralization on the ferrous ion bio-oxidation process during acid mine drainage treatment. J Hazard Mater 299:404–411
Machodi MJ, Daramola MO (2019) Synthesis and performance evaluation of PES/chitosan membranes coated with polyamide for acid mine drainage treatment. Sci Rep 9:17657
Moreno-González R, Cánovas CR, Olías M, Macías F (2020) Seasonal variability of extremely metal rich acid mine drainages from the Tharsis mines (SW Spain). Environ Pollut 259:113829
Naidu G, Ryu S, Thiruvenkatachari R, Choi Y, Jeong S, Vigneswaran S (2019) A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ Pollut 247:1110–1124
Ngegla JV, Zhou X, Chen X, Zhu X, Liu Z, Feng J, Zeng XC (2020) Unique diversity and functions of the arsenic-methylating microorganisms from the tailings of Shimen Realgar Mine. Ecotoxicology 29:86–96
Ozoko DC (2015) Heavy metal geochemistry of acid mine drainage in Onyeama Coal Mine, Enugu, Southeastern Nigeria. J Environ Earth Sci 5:120–127
Park I, Tabelin CB, Jeon S, Li X, Seno K, Ito M, Hiroyoshi N (2019) A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 219:599–606
Shi W, Wu W, Zeng XC, Chen X, Zhu X, Cheng S (2018) Dissimilatory arsenate-respiring prokaryotes catalyze the dissolution, reduction and release of arsenic from paddy soils into groundwater: implication for the effect of sulfate. Ecotoxicology 27:1126–1136
Sun J, Hong Y, Guo J, Yang J, Huang D, Lin Z, Jiang F (2019) Arsenite removal without thioarsenite formation in a sulfidogenic system driven by sulfur reducing bacteria under acidic conditions. Water Res 151:362–370
Sun R, Zhang L, Zhang Z, Chen GH, Jiang F (2018) Realizing high-rate sulfur reduction under sulfate-rich conditions in a biological sulfide production system to treat metal-laden wastewater deficient in organic matter. Water Res 131:1343–1354
Sun W, Sierra-Alvarez R, Field JA (2011) Long term performance of an arsenite-oxidizing-chlorate-reducing microbial consortium in an upflow anaerobic sludge bed (UASB) bioreactor. Bioresour Technol 102:5010–5016
Villegas-Plazas M, Sanabria J, Junca H (2019) A composite taxonomical and functional framework of microbiomes under acid mine drainage bioremediation systems. J Environ Manage 251:109581
Wang J, Zeng XC, Zhu X, Chen X, Zeng X, Mu Y, Yang Y, Wang Y (2017) Sulfate enhances the dissimilatory arsenate-respiring prokaryotes-mediated mobilization, reduction and release of insoluble arsenic and iron from the arsenic-rich sediments into groundwater. J Hazard Mater 339:409–417
Wang X, Jiang H, Fang D, Liang J, Zhou L (2019) A novel approach to rapidly purify acid mine drainage through chemically forming schwertmannite followed by lime neutralization. Water Res 151:515–522
Yang Y, Mu Y, Zeng XC, Wu W, Yuan J, Liu Y,E,G, Luo F, Chen X, Li H, Wang J (2017) Functional genes and thermophilic microorganisms responsible for arsenite oxidation from the shallow sediment of an untraversed hot spring outlet. Ecotoxicology 26:490–501
Zeng XC,E,G, Wang J, Wang N, Chen X, Mu Y, Li H, Yang Y, Liu Y, Wang Y (2016) Functions and Unique Diversity of Genes and Microorganisms Involved in Arsenite Oxidation from the Tailings of a Realgar Mine. Appl Environ Microbiol 82:7019–7029
Zeng XC, Yang Y, Shi W, Peng Z, Chen X, Zhu X, Wang Y (2018) Microbially Mediated Methylation of Arsenic in the Arsenic-Rich Soils and Sediments of Jianghan Plain. Front Microbiol 6:1389
Zhang X, Tang S, Wang M, Sun W, Xie Y, Peng H, Zhong A, Zhang X, Yu H, Giesy JP, Hecker M (2019) Acid mine drainage affects the diversity and metal resistance gene profile of sediment bacterial community along a river. Chemosphere 217:790–799
Zhu X, Zeng XC, Chen X, Wu W, Wang Y (2019) Inhibitory effect of nitrate/nitrite on the microbial reductive dissolution of arsenic and iron from soils into pore water. Ecotoxicology 28:528–538
Acknowledgements
This work was supported by the General Programs and the Foundations for Innovative Research Groups from the National Natural Science Foundation of China (grant nos. 41472219 and 41521001), and the Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) (grant no. CUGCJ1702).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Xu, Y., Li, H. & Zeng, XC. A novel biofilm bioreactor derived from a consortium of acidophilic arsenite-oxidizing bacteria for the cleaning up of arsenite from acid mine drainage. Ecotoxicology 30, 1437–1445 (2021). https://doi.org/10.1007/s10646-020-02283-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02283-4