Abstract
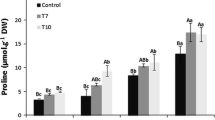
Proline is one of the most important compatible osmolyte in cells, which accumulates in response to various stresses, including salt, water deficit, heavy metal, pathogen infection and extreme temperature. In this study, a growth chamber was employed to simulate heat environment for Avicennia marina seedlings. We detected some physiological indices in the leaves of A. marina at 40 °C, including the activity of delta-1-pyrroline-5-carboxylate synthase (P5CS), the content of free proline and soluble protein, transpiration rate and membrane permeability, and discussed the relationship between these five indices and heat resistant ability. And then a P5CS gene was cloned from A. marina using homologous cloning and rapid amplification of cDNA ends methods. It was designated as AmP5CS, encoding a protein that contained a feedback inhibition site of proline, proA, proB, conserved Leu zipper, GSA-DH domain and other functional domains of P5CS protein in high plants. Expression analysis of AmP5CS gene indicated it was involved in heat stress response. It is the first time that P5CS from A. marina has been cloned and the findings laid the foundation of figuring out heat resistant mechanisms and relieving heat damage, which is significant during global warming.







Similar content being viewed by others
References
Alongi DM (2015) The impact of climate change on mangrove forests. Curr Clim Change Rep 1:30–39
Atienza SG, Faccioli P, Perrotta G, Dalfino G, Zschiesche W, Humbeck K, Stanca AM, Cattivelli L (2004) Large scale analysis of transcripts abundance in barley subjected to several single and combined abiotic stress conditions. Plant Sci 167(6):1359–1365
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Blum A (2018) Plant breeding for stress environments. CRC press, Boca Raton, FL
Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat1. Crop Sci 21(1):43–47
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72:248–254
Charest C, TonPhan C (1990) Cold acclimation of wheat (Triticum aestivum): properties of enzymes involved in proline metabolism. Physiol Plant 80(2):159–168
Chen J, Zhang X, Jing R, Blair MW, Mao X, Wang S (2010) Cloning and genetic diversity analysis of a new P5CS gene from common bean (Phaseolus vulgaris L.). Theor Appl Genet 120(7):1393–1404
Chen JB, Wang SM, Jing RL, Mao XG (2009) Cloning the PvP5CS gene from common bean (Phaseolus vulgaris) and its expression patterns under abiotic stresses. J Plant Physiol 166(1):12–19
Chou IT, Chen CT, Kao CH (1991) Characteristics of the induction of the accumulation of proline by abscisic acid and isobutyric acid in detached rice leaves. Plant Cell Physiol 32(2):269–272
Csonka LN (1989) Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev 53(1):121–147
Delauney AJ, Verma DS (1993) Proline biosynthesis and osmoregulation in plants. Plant J 4(2):215–223
Elthon TE, Stewart CR (1981) Submitochondrial location and electron transport characteristics of enzymes involved in proline oxidation. Plant Physiol 67(4):780–784
Faw WF, Shih SC, Jung GA (1976) Extractant influence on the relationship between extractable proteins and cold tolerance of alfalfa. Plant Physiol 57(5):720–723
Fei J, Wang YS, Zhou Q, Gu JD (2015) Cloning and expression analysis of HSP70 gene from mangrove plant Kandelia obovata under cold stress. Ecotoxicology 24(7-8):1677–1685
Fujita T, Maggio A, Garcia-Rios M, Bressan RA, Csonka LN (1998) Comparative analysis of the regulation of expression and structures of two evolutionarily divergent genes for Δ1-pyrroline-5-carboxylate synthetase from tomato. Plant Physiol 118(2):661–674
Gilman EL, Ellison J, Duke NC, Field C (2008) Threats to mangroves from climate change and adaptation options: a review. Aquat Bot 89:237–250
Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF (2000) Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol 124(4):1854–1865
Ginzberg I, Stein H, Kapulnik Y, Szabados L, Strizhov N, Schell J, Koncz C, Zilberstein A (1998) Isolation and characterization of two different cDNAs of Δ1-pyrroline-5-carboxylate synthase in alfalfa, transcriptionally induced upon salt stress. Plant Mol Biol 38(5):755–764
Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21(2):79–102
Hayzer DJ, Leisinger TH (1980) The gene-enzyme relationships of proline biosynthesis in Escherichia coli. Microbiology 118(2):287–293
Hmida-Sayari A, Gargouri-Bouzid R, Bidani A, Jaoua L, Savouré A, Jaoua S (2005) Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers salt tolerance in transgenic potato plants. Plant Sci 169(4):746–752
Hong Z, Lakkineni K, Zhang Z, Verma DS (2000) Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122(4):1129–1136
Hu CA, Delauney AJ, Verma DP (1992) A bifunctional enzyme (delta1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis in plants. Proc Natl Acad Sci 89(19):9354–9358
Huber B (1927) Zur Methodik der Transpirationsbestimmung am Standort. Ber Dtsch Bot Ges 45:611–618
Igarashi Y, Yoshiba Y, Sanada Y, Yamaguchi-Shinozaki K, Wada K, Shinozaki K (1997) Characterization of the gene for Δ1-pyrroline-5-carboxylate synthetase and correlation between the expression of the gene and salt tolerance in Oryza sativa L. Plant Mol Biol 33(5):857–865
Jung Y, Park J, Choi Y, Yang JG, Kim D, Kim BG, Roh K, Lee DH, Auh CK, Lee S (2010) Expression analysis of proline metabolism-related genes from halophyte Arabis stelleri under osmotic stress conditions. J Integr Plant Biol 52(10):891–903
Khedr AHA, Abbas MA, Wahid AAA, Quick WP, Abogadallah GM (2003) Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt‐stress. J Exp Bot 54(392):2553–2562
Kishor PK, Hong Z, Miao GH, Hu CA, Verma DS (1995) Overexpression of [delta]-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108(4):1387–1394
Kuznetsov VV, Shevyakova NI (1999) Proline under stress: biological role, metabolism, and regulation. Rus J Plant Physiol 46(2):274–287
Leisinger T (1987) Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington D.C., USA
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Livné A, Vaadia Y (1965) Stimulation of transpiration rate in barley leaves by kinetin and gibberellic acid. Physiol Plant 18(3):658–664
Lovelock CE, Cahoon DR, Friess DA, Guntenspergen GR, Krauss KW, Reef R, Rogers K, Saunders ML, Sidik F, Swales A, Saintilan N, Thuyen LX, Triet T (2015) The vulnerability of Indo-Pacific mangrove forests to sea-level rise. Nature 526:559–563
Machado S, Paulsen GM (2001) Combined effects of drought and high temperature on water relations of wheat and sorghum. Plant Soil 233(2):179–187
Mahajan PV, Oliveira FR, Macedo I (2008) Effect of temperature and humidity on the transpiration rate of the whole mushrooms. J Food Eng 84(2):281–288
Molinari HBC, Marur CJ, Bespalhok Filho JC, Kobayashi AK, Pileggi M, Júnior RL, Pereira LP, Vieira LE (2004) Osmotic adjustment in transgenic citrus rootstock Carrizo citrange (Citrus sinensis Osb. x Poncirus trifoliata L. Raf.) overproducing proline. Plant Sci 167(6):1375–1381
Nathalie V, Christian H (2008) Proline accumulation in plants: A review. Amino Acids 35:753–759
Paleg LG, Stewart GR, Bradbeer JW (1984) Proline and glycine betaine influence protein solvation. Plant Physiol 75(4):974–978
Parvanova D, Ivanov S, Konstantinova T, Karanov E, Atanassov A, Tsvetkov T, Alexieva V, Djilianov D (2004) Transgenic tobacco plants accumulating osmolytes show reduced oxidative damage under freezing stress. Plant Physiol Biochem 42(1):57–63
Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) (2018) Climate change 2013: the physical science basis. Cambridge University Press, Cambridge, UK
Rayapati PJ, Stewart CR, Hack E (1989) Pyrroline-5-carboxylate reductase is in pea (Pisum sativum L.) leaf chloroplasts. Plant Physiol 91(2):581–586
Savouré A, Jaoua S, Hua XJ, Ardiles W, Van Montagu M, Verbruggen N (1995) Isolation, characterization, and chromosomal location of a gene encoding the Δ1-pyrroline-5-carboxylate synthetase in Arabidopsis thaliana. FEBS Lett 372(1):13–19
Schobert B, Tschesche H (1978) Unusual solution properties of proline and its interaction with proteins. Biochim Biophys Acta 541(2):270–277
Silva-Ortega CO, Ochoa-Alfaro AE, Reyes-Agüero JA, Aguado-Santacruz GA, Jiménez-Bremont JF (2008) Salt stress increases the expression of p5cs gene and induces proline accumulation in cactus pear. Plant Physiol Biochem 46(1):82–92
Siripornadulsil S, Traina S, Verma DS, Sayre RT (2002) Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 14(11):2837–2847
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochem 28(4):1057–1060
Solomon A, Beer S, Waisel Y, Jones GP, Paleg LG (1994) Effects of NaCl on the carboxylating activity of Rubisco from Tamarix jordanis in the presence and absence of proline-related compatible solutes. Physiol Plant 90(1):198–204
Song SQ, Lei YB, Tian XR (2005) Proline metabolism and cross-tolerance to salinity and heat stress in germinating wheat seeds. Rus J Plant Physiol 52(6):793–800
Strizhov N, Ábrahám E, Ökrész L, Blickling S, Zilberstein A, Schell J, Koncz C, Szabados L (1997) Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J 12(3):557–569
Su M, Li XF, Ma XY, Peng XJ, Zhao AG, Cheng LQ, Chen SY, Liu GS (2011) Cloning two P5CS genes from bioenergy sorghum and their expression profiles under abiotic stresses and MeJA treatment. Plant Sci 181(6):652–659
Szoke A, Miao GH, Hong Z, Verma DS (1992) Subcellular location of δ1-pyrroline-5-carboxylate reductase in root/nodule and leaf of soybean. Plant Physiol 99(4):1642–1649
Tripathy JN, Zhang J, Robin S, Nguyen TT, Nguyen HT (2000) QTLs for cell-membrane stability mapped in rice (Oryza sativa L.) under drought stress. Theor Appl Genet 100(8):1197–1202
Wang LY, Wang YS, Zhang JP, Gu JD (2015) Molecular cloning of class III chitinase gene from Avicennia marina and its expression analysis in response to cadmium and lead stress. Ecotoxicology 24(7–8):1697–1704
Wang YS (2019) Molecular ecology of mangroves. The Science Publishing Company, Beijing, China
Yoshiba Y, Kiyosue T, Katagiri T, Ueda H, Mizoguchi T, Yamaguchi-Shinozaki K, Wada K, Harada Y, Shinozaki K (1995) Correlation between the induction of a gene for Δ1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. Plant J 7(5):751–760
Yurekli F, Porgali ZB, Turkan I (2004) Variations in abscisic acid, indole-3-acetic acid, gibberellic acid and zeatin concentrations in two bean species subjected to salt stress. Acta Biol Crac Ser Bot 46:201–212
Zhang C, Lu Q, Verma DS (1995) Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase, a bifunctional enzyme catalyzing the first two steps of proline biosynthesis in plants. J Biol Chem 270(35):20491–20496
Zhang CS, Lu Q, Verma DS (1995) Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase, a bifunctional enzyme catalyzing the first two steps of proline biosynthesis in plants. J Biol Chem 270(35):20491–20496
Zhu XY, Li XP, Zou Y, Chen WX, Lu WJ (2012) Cloning, characterization and expression analysis of Δ1-pyrroline-5-carboxylate synthetase (P5CS) gene in harvested papaya (Carica papaya) fruit under temperature stress. Food Res Int 49(1):272–279
Acknowledgements
This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA23050200 and No. XDA13010500), the National Natural Science Foundation of China (No.U1901211, No. 41430966 and No. 41876126), the National Key Research and Development Plan (No. 2017FY100700) and the International Partnership Program of Chinese Academy of Sciences (No. 133244KYSB20180012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All the related authors have known the informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, J., Wang, YS. Proline metabolism and molecular cloning of AmP5CS in the mangrove Avicennia marina under heat stress. Ecotoxicology 29, 698–706 (2020). https://doi.org/10.1007/s10646-020-02198-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02198-0